Volume 8 (2025) Issue 4 No.2 Pages 262-267
Abstract
In haploidentical peripheral blood stem cell transplantation with post-transplant cyclophosphamide (PTCy-haplo), early initiation of calcineurin inhibitors may affect transplant outcome by inducing CD8+ transient exhaustion-like T cells which are important for chronic graft-versus-host disease and graft-versus-leukemia/lymphoma effects based on a murine model. However, to our best knowledge, there has been no report about modification of fludarabine (Flu)/melphalan (Mel)-based PTCy-haplo in a real-world setting. To evaluate the impact of early tacrolimus (TAC) initiation on outcomes, we retrospectively analyzed 19 patients at our institution between April 2017 and March 2024. The incidence and grade of cytokine release syndrome (CRS) were low (31.6% and all grades ≤ 2). In addition, despite the poor baseline characteristics including older age (median, 59 years [range, 25-72]), active diseases at the time of PTCy-haplo (n=8), and short interval between the first and second allogeneic hematopoietic stem cell transplantation (allo-HSCT) (n=8; median, 12.4 months [range, 3.9-32.9]; all patients received Flu/busulfan (Bu)-based conditioning regimen for their first allo-HSCT), only two patients (10.5%) developed sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD). With a median follow-up of 30.2 months (range, 1.0-53.1), overall survival and disease-free at 2 years were 56.8 and 51.5%, respectively. These findings suggested that early TAC initiation using Flu/Mel-based PTCy-haplo may be potentially useful for older patients with low tolerance to CRS, high risk of SOS/VOD or recurrence, or for those who are unlikely to receive Bu-based conditioning regimen due to early relapse after allo-HSCT with Bu-based conditioning regimen.
Introduction
The use of post-transplant cyclophosphamide (PTCy) as graft-versus-host disease (GVHD) prophylaxis widely spread because of its high efficacy in reducing GVHD and low incidence of non-relapse mortality (NRM). However, the data of efficacy and safety of the fludarabine (Flu)/melphalan (Mel) as conditioning regimen for haploidentical-peripheral blood stem cell transplantation with PTCy (PTCy-haplo) in clinical practice are lacking. Recently, Koller et al. reported a median age of 61 years with a 100 mg/m2 dose of Mel (if patients were
Early initiation of calcineurin inhibitors increases chronic GVHD by inducing CD8+ transient exhaustion-like T cells (Tex) and granzyme B+ CD4+ T cells3. These transitory Tex are also critical for graft-versus-leukemia/lymphoma effects in murine model4. To clarify the relationship between the timing of tacrolimus (TAC) initiation and transplant outcomes, we have previously attempted to modify PTCy-haplo by initiating TAC (day 5 to −1) in patients with high risk of NRM or relapse5. In this study, overall survival (OS), CIR, and NRM were similar in both groups, but early TAC initiation in combination with the reduced-dose PTCy (80 mg/kg) lead to an increase in chronic GVHD. In addition, other previous studies have reported that these modifications of PTCy-haplo may contribute to improved incidence of cytokine release syndrome (CRS), and disease-free survival (DFS)6,7; however, these previous reports were mainly based on the Flu/Bu-based conditioning regimen, and to the best of our knowledge, the data regarding modification of GVHD prophylaxis in the Flu/Mel-based conditioning regimen is lacking. Given the widespread PTCy-haplo and increasing reports on Flu/Mel-based conditioning regimen1,2, the current study focused on patients receiving Flu/Mel-based reduced intensity conditioning regimen to evaluate the efficacy and safety of early TAC initiation.
Methods
Patient selection
We performed a retrospective analysis of consecutive patients over 16 years old with hematopoietic neoplasms who received Flu/Mel-based PTCy-haplo at our institution between April 2017 and March 2024 (
Conditioning regimen and GVHD prophylaxis
All patients received conditioning regimens based on five doses of fludarabine at 30 mg/m2 on day −6 to −2, two doses of melphalan at 40-60 mg/m2 on day −3 to day −2, and a one or two dose of total body irradiation at 2 Gy, followed by haploidentical peripheral blood stem cell transplantation per institutional protocols.
As GVHD prophylaxis, TAC (0.02 mg/kg with the dose being modified to target serum trough levels of 10-12 ng/mL) was initiated on day −1, followed by Cy (40-50 mg/kg) on day 3 to 4 and mycophenolate mofetil (MMF; 15 mg/kg orally twice daily with a maximum daily dose of 2,000 mg) from day 5. The modification of TAC (i.e. day 5 to −1) was mainly decided by the treating physicians based on the following principles: patients with older, other comorbidities, or a high risk of relapse. MMF was tapered off beginning on day 30 after PTCy-haplo. After discontinuation of MMF, the duration of TAC was primarily determined by the attending physician, considering the presence of GVHD and the risk of recurrence.
Definitions
Details are described in
Statistical analysis
The Mann-Whitney U test was used to compare continuous variables, and categorical variables were compared using Fisher's exact test. The probabilities of OS, DFS, and graft-versus-host/relapse-free survival (GRFS) were estimated using the Kaplan-Meier method. The probabilities of grade II-IV and III-IV acute GVHD, moderate to severe chronic GVHD, relapse, and NRM were estimated based on cumulative incidence methods and compared using Gray's test. Competing events included relapse or death without neutrophil and platelet engrafment for engraftment, relapse or death without GVHD for acute and chronic GVHD, relapse for NRM, and NRM for relapse. All statistical analyses were performed using EZR version 1.65 (Saitama Medical Center, Jichi Medical University, Saitama, Japan)8.
Results
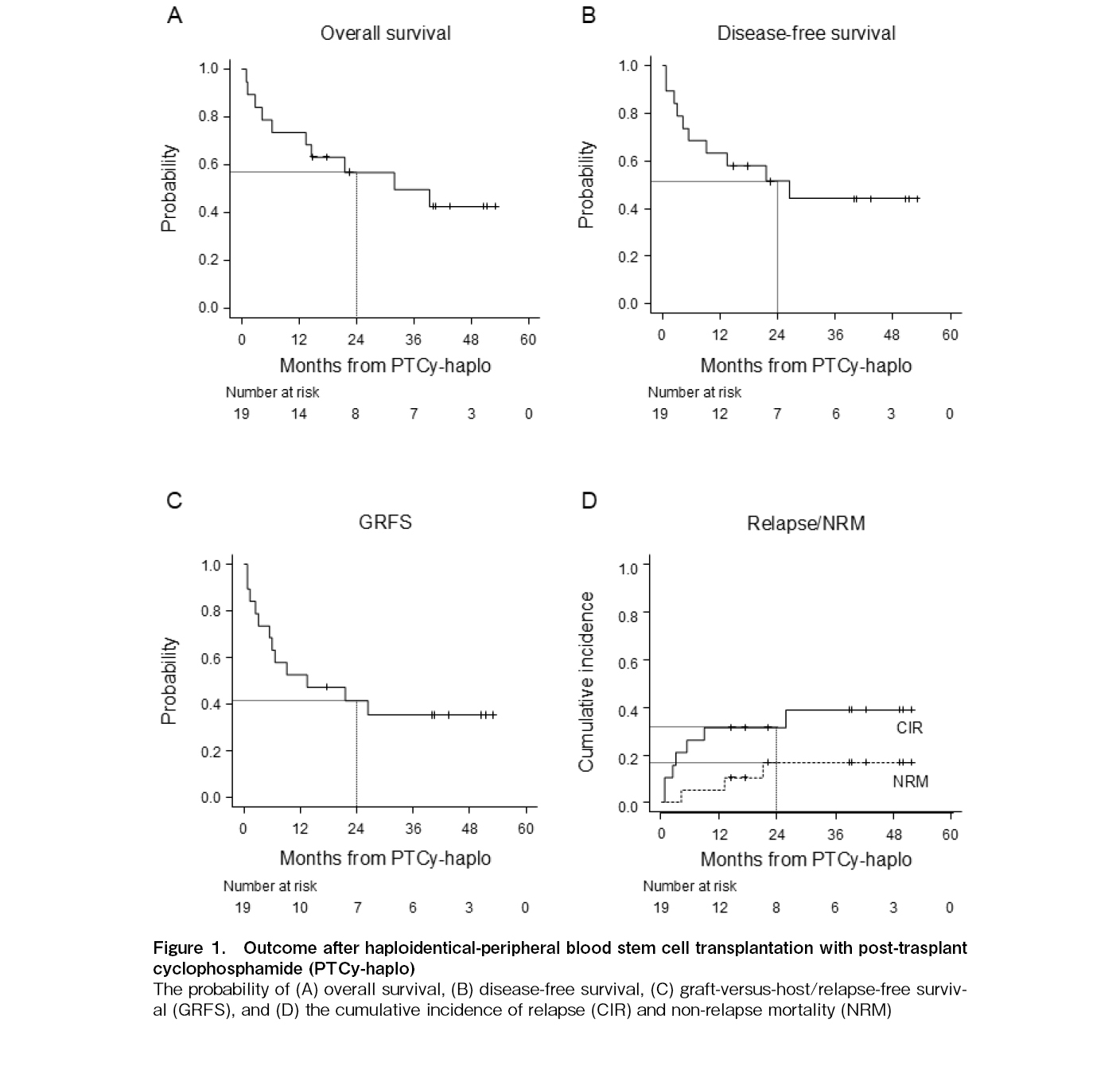
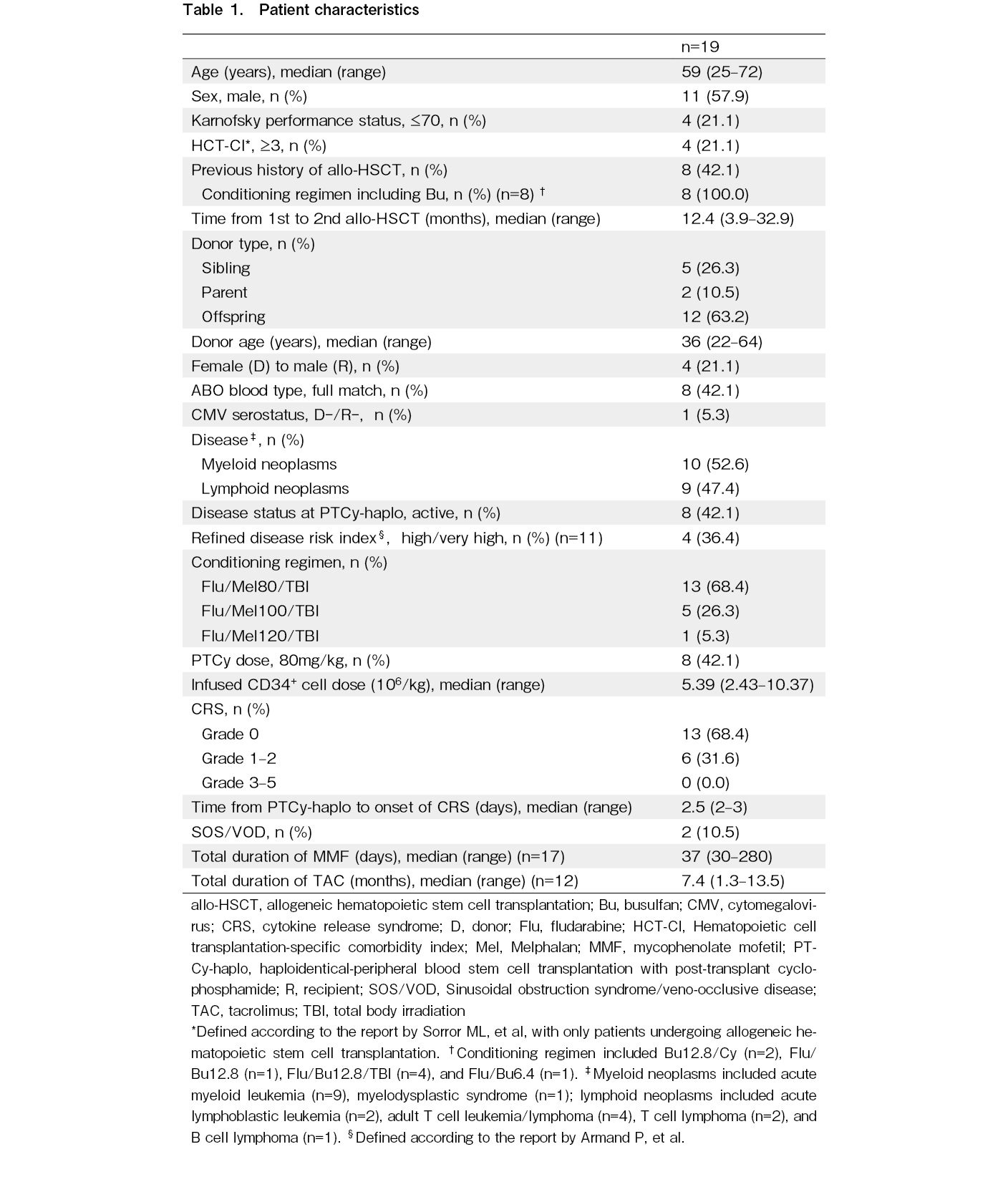
Nineteen patients were included in this study and patient characteristics were shown in Table 1. The median patient age was 59 years (range, 25-72). Eleven male donors (57.9%) were included. Two patients had Karnofsky performance (KPS) status ≤ 70 and hematopoietic cell transplantation-specific comorbidity index ≥ 3, and four patients met either factor. Eight patients (42.1%) had previous history of allogeneic hematopoietic stem cell transplantation (allo-HSCT), with a median time to second transplant of 12.4 months (range, 3.4-32.9). All patients received conditioning regimen including Bu at the time of their first allo-HSCT. Eight patients (42.1%) had active diseases at the time of PTCy-haplo and 4 out of 11 patients (36.4%) undergoing their first transplant had refined disease risk index (rDRI) ≥ high. In eight patients, PTCy dose was reduced (i.e. 40 mg/kg/day × 2 days) based on the discretion of the treating physician for the following reasons: seven patients received their second transplant, and one patient was elderly (72 years).
Six patients (31.6%) had mild (grade 1-2) CRS, with a median of 2.5 days after PTCy-haplo and none developed severe CRS (grade ≥ 3). Except for two patients who relapsed early after PTCy-haplo, all patients were engrafted, and the median duration of neutrophil and platelet engraftment was 14 and 24 days, respectively (
Discussion
In this study, we evaluated the clinical impact of the early TAC initiation in patients with haplo-HSCT with PTCy and Flu/Mel-based conditioning regimen. Although comparisons across the different studies require careful interpretation due to potentially significant unrecognized limitations, our data revealed that despite a higher proportion of patients with KPS ≤ 70 (21.1 versus (vs.) 11.9%), 2-year NRM was lower as compared to the largest previous study of patients received Flu/Mel-based conditioning regimen using PTCy to our best knowledge so far (16.8 vs. approximately 30%)1. Furthermore, our results showed similar outcomes (2-year OS, 56.8 vs. 58.0%; 2-year DFS, 51.5 vs. 52.0%), but a higher 2-year CIR (31.6 vs. approximately 20%), because this study included patients considered to be at relatively high risk of recurrence for the following reasons: 1) a slightly higher proportion of refined disease risk index (only 1st transplant patients) ≥ high patients (36.4 vs. 29.8%), and 2) approximately 40% of patients received a second transplant (not described by Koller et al.1). In contrast, 1-year cumulative incidence of moderate to severe chronic GVHD was high, resulting in slightly inferior 2-year GRFS (41.4 vs. 48.0%). The increased cumulative incidence of moderate-to-severe chronic GVHD was particularly notable in the reduced-dose PTCy group, and we assumed that both modification were contributed to attenuated effect of PTCy on allogeneic reactive T cell depletion3,5. The maintained survival of these alloreactive T cell clones has been suggested to potentially enhanced the graft-versus-leukemia/lymphoma (GVL) effects in murine model4, but it is important to consider the potential trade-off between enhanced the GVL effects and increased moderate-to-severe chronic GVHD. The incidence of SOS/VOD, which was the representative serious complication after Bu-based conditioning regimen, was slightly higher compared to previous report of PTCy-haplo settings10, possibly due to the worse baseline parameters including older age and short interval between the first and second allo-HSCT. With regard to infections, previous reports have shown a trend toward fewer cases of bacterial infection and BK virus hemorrhagic cystitis in the reduced-dose PTCy group11,12, but this study did not find such a tendency. This may be due to the small sample size and heterogeneity patient background. Taken together, our results imply that Flu/Mel-based conditioning regimen with early TAC initiation may be potentially useful for the following patients: 1) older patients with low tolerance to CRS, 2) patients with a high risk of SOS/VOD or recurrence, or 3) patients who had early relapse after Bu-based conditioning regimen. However, we fully acknowledge that further comparative studies with standard TAC initiation (i,e, day 5) or Bu-based conditioning regimen are warranted to determine whether this treatment strategy could be a safe and feasible option for these patients.
The current study limited its retrospective nature and a small group of heterogeneous patients. Approximately 40% of patients underwent their second transplant, or had active disease at the time of PTCy-haplo. Furthermore, since it has been reported that PTCy dose reduction is associated with a decrease in NRM13, these factors had a potential impact on our findings independently of early TAC initiation. However, this is the first study to investigate the efficacy and safety of modified Flu/Mel-based PTCy-haplo in a real-world setting and our data will be the cornerstone of a future study evaluating GVHD prophylaxis in Flu/Mel-based conditioning regimen with PTCy-haplo.
In conclusion, optimizing GVHD prophylaxis strategies in Flu/Mel-based conditioning regimens for PTCy-haplo according to patient background may be important, especially in patients considered unlikely to use Bu-based conditioning regimen. Further prospective studies with larger sample sizes are required to validate our findings.
Acknowledgments
We thank all the patients who participated in this study. The manuscript was edited and proofread by Editage (
Author Contributions
WK and TT performed conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, and writing the original draft. K-IM, TK, KS, KF, HF, NA DE, and NF performed review and editing. YM performed supervision, review, and editing. All authors approved the final manuscript. WK and TT contributed equally to this work.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website. K-IM is one ot the Editors of Blood Cell Therapy. He was not involved in the editorial evaluation or decision to accept this article for publication.
Acknowledgments
We thank all the patients who participated in this study. The manuscript was edited and proofread by Editage (
Data Availability
The clinical data collected for this study are maintained by authors and may be shared upon reasonable request.
References
1.Koller PB, Othman T, Yang D, Mokhtari S, Samara Y, Blackmon A, et al. Melphalan-based conditioning with post-transplant cyclophosphamide for peripheral blood stem cell transplantation: donor effect. Bone Marrow Transplant. 2025; 50: 625-31.
2.Eastburg L, Russler-Germain DA, DiPersio JF, Fountaine T, Andolina JR, Abboud R, et al. Increased early mortality after fludarabine and melphalan conditioning with peripheral blood grafts in haploidentical hematopoietic cell transplantation with post-transplant cyclophosphamide. Leuk Lymphoma. 2022; 63: 222-6.
3.Senjo H, Harada S, Kubota SI, Tanaka Y, Tateno T, Zhang Z, et al. Early Initiation of Calcineurin Inhibitor Induces CD8+ Transitory Exhausted-like and CD4+ Cytotoxic T Cells, Maintaining Responsiveness to PD-1 Blockade and Contributing to the Developmet of Chronic Gvhd after Post-Transplant Cyclophosphamide. Blood. 2024; 144 (Suppl 1): 903.
4.Senjo H, Harada S, Kubota SI, Tanaka Y, Tateno T, Zhang Z, et al. Calcineurin inhibitor inhibits tolerance induction by suppressing terminal exhaustion of donor T cells after allo-HCT. Blood. 2023; 142: 477-92.
5.Terao T, Kondo T, Nakamura M, Takasuka H, Fujiwara H, Asada N, et al. Combination of reduced post-transplant cyclophosphamide and early tacrolimus initiation increases the incidence of chronic graft-versus-host disease in human leukocyte antigen-haploidentical peripheral blood stem-cell transplantation. EJHaem. 2024; 5: 810-4.
6.Ruggeri A, Labopin M, Battipaglia G, Chiusolo P, Tischer J, Diez-Martin JL, et al. Timing of Post-Transplantation Cyclophosphamide Administration in Haploidentical Transplantation: A Comparative Study on Behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2020; 26: 1915-22.
7.Kurita N, Sakamoto T, Kato T, Kusakabe M, Yokoyama Y, Nishikii H, et al. Early administration of cyclosporine may reduce the incidence of cytokine release syndrome after HLA-haploidentical hematopoietic stem-cell transplantation with post-transplant cyclophosphamide. Ann Hematol. 2021; 100: 1295-301.
8.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR' for medical statistics. Bone Marrow Transplant. 2013; 48: 452-8.
9.Mohty M, Malard F, Alaskar AS, Aljurf M, Arat M, Bader P, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a refined classification from the European society for blood and marrow transplantation (EBMT). Bone Marrow Transplant. 2023; 58: 749-54.
10.Balaguer-Rosello A, Montoro J, Villalba M, Lizama P, Torres L, Chorão P, et al. Sinusoidal Obstruction Syndrome After Allogeneic Hematopoietic Cell Transplantation With Post-Transplant Cyclophosphamide. Transplant Cell Ther. 2025; 31: 674.e1-674.e13.
11.Duléry R, Goudet C, Mannina D, Bianchessi A, Granata A, Harbi S, et al. Reduced post-transplant cyclophosphamide doses in haploidentical hematopoietic cell transplantation for elderly patients with hematological malinancies. Bone Marrow Transplant. 2023; 58: 386-92.
12.Fuji S, Sugita J, Najima Y, Konishi T, Tanak T, Ohigashi H, et al. Low-versus strandard-dose post-transplant cyclophosphamide as GVHD prophylaxis for haploidentical transplantation. Br J Haematol. 2024; 204: 959-66.
13.Negrão de Araujo TF, Rocha LPS, de Iliveira CF, de Morais Ferreira CF, de Souza BA, Puls ML, et al. Impact of Reduced-Dose of Post-Transplantation Cyclophosphamide (PTCy) in HLA-Identical and Haploidentical Hematopoietic Stem Cell Transplantation: A Retrospective Analysis. Blood. 2024; 144 (Suppl 1): 7324.
Search
News