Volume 8 (2025) Issue 4 No.3 Pages 268-273
Abstract
The Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway is crucial for immune function and hematopoiesis, which partly explains why cytopenia is a major adverse effect of ruxolitinib, a selective JAK1/2 inhibitor. Hematologic toxicity restricts the use of ruxolitinib during the pre-engraftment phase of allogeneic hematopoietic stem cell transplantation for myelofibrosis and graft-versus-host disease (GVHD) prophylaxis. While some reports have described its use in peripheral blood and bone marrow transplantation, its application in cord blood transplantation (CBT) remains unknown. Herein, we report two cases of CBT in which ruxolitinib was administered to treat GVHD after prior allogeneic transplantation. In Case 1, a patient underwent a third transplant for acute myeloid leukemia, and in Case 2, a patient received CBT for post-transplant lymphoproliferative disorder following transplantation for classic Hodgkin lymphoma. Neutrophil engraftment was achieved in both cases, and Case 2 developed a pre-engraftment immune reaction. Platelet and red blood cell engraftment did not occur in either case, likely due to underlying comorbidities or limited survival, rather than the effects of ruxolitinib. This is the first report documenting successful neutrophil engraftment in CBT with concurrent ruxolitinib administration, suggesting its potential feasibility during the pre-engraftment phase. Further studies are warranted to evaluate its effects on multilineage hematopoietic recovery, infections, GVHD, and relapse risk.
Introduction
The Janus kinases (JAK)-signal transducer and activator of transcription (STAT) pathway plays crucial roles in both the immune and hematopoietic systems1. Ruxolitinib, a selective JAK1/2 inhibitor, was initially approved for the treatment of myelofibrosis and subsequently for both acute and chronic graft-versus-host disease (GVHD)2,3. Cytopenia is one of its most frequently reported adverse effects during treatment for either condition4–7.
Ruxolitinib has shown efficacy during allogeneic hematopoietic stem cell transplantation (AlloSCT) for the management of myelofibrosis and prophylaxis of GVHD8,9. Despite the hematopoietic toxicity of ruxolitinib, successful engraftment has been reported. In these cases, patients underwent peripheral blood stem cell transplantation (PBSCT) or bone marrow transplantation, both of which typically involve the infusion of a higher number of stem cells than umbilical cord blood transplantation (CBT). To our knowledge, no reports have documented successful engraftment with concurrent ruxolitinib administration in CBT. In this report, we present two cases of successful neutrophil engraftment following CBT with concurrent ruxolitinib administration, along with a review of the literature on ruxolitinib use in stem cell transplantation.
Case Presentation
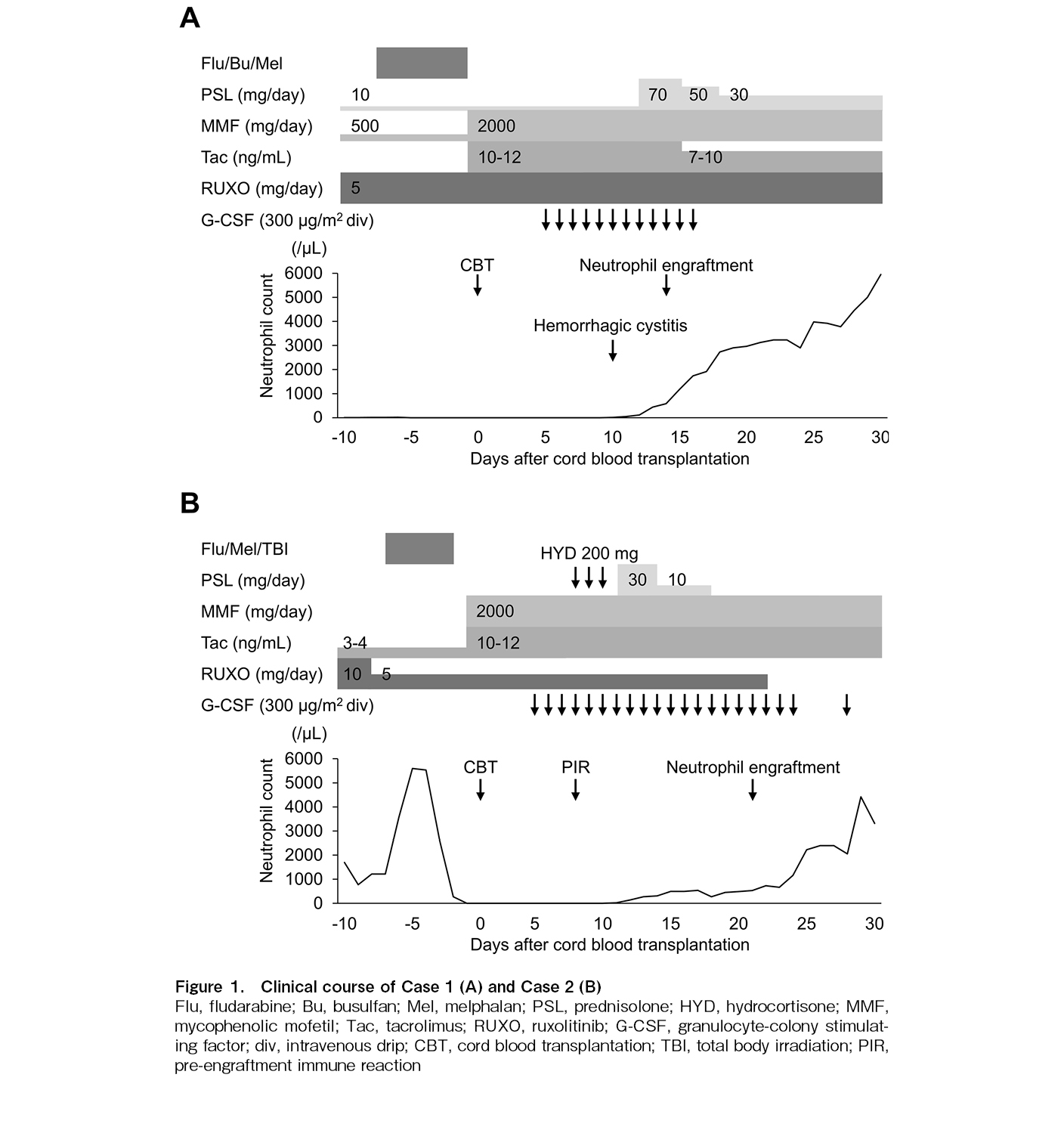
Case 1 (Figure 1A): A 45-year-old woman with acute myeloid leukemia (AML) experienced two relapses following AlloSCT and was scheduled to undergo a third AlloSCT. After the second AlloSCT, she was treated for acute GVHD with ruxolitinib (10 mg/day), prednisolone, cyclosporine, and mycophenolate mofetil (MMF). She received salvage therapy and underwent a single-unit CBT. The conditioning regimen consisted of fludarabine (180 mg/m2), busulfan (9.6 mg/kg), and melphalan (80 mg/m2). The total number of infused nucleated cells was 1.7 × 107/kg (CD34-positive cells: 0.35 × 105/kg). Human leukocyte antigen (HLA) was mismatched at two of the six antigens in the graft-versus-host direction and at one antigen in the host-versus-graft direction, with three of the eight alleles mismatched in both directions. No donor-specific antibody was detected. Tacrolimus and MMF were administered for GVHD prophylaxis. Due to ongoing diarrhea caused by acute GVHD from the second AlloSCT, both prednisolone and ruxolitinib were continued during initial conditioning. The ruxolitinib dose was reduced to 5 mg/day due to the anticipated immunosuppressive effects of the conditioning regimen and concerns regarding the potential hematopoietic toxicity of ruxolitinib.
On day 10, she developed severe hemorrhagic cystitis owing to BK virus infection. On day 12, prednisolone was increased as hemophagocytic syndrome was suspected. Neutrophil engraftment, defined as a neutrophil count exceeding 500/μL, was achieved on day 14. On day 28, short tandem repeat (STR) analysis of peripheral blood revealed complete donor chimerism in both granulocytes and T cells. Reticulocyte count exceeded 1.0% of the total erythrocytes by day 31. She developed idiopathic pneumonia syndrome following the dose reduction of MMF and tacrolimus. Concurrently, she developed transplant-associated thrombotic microangiopathy (TA-TMA)-induced fragmented red cells, leading to the discontinuation of tacrolimus; nonetheless, TA-TMA did not improve. The patient experienced increased bleeding due to BK virus cystitis, which was further complicated by BK viremia. Although there was no evidence of AML relapse, she died on day 102 owing to multiple organ failure. Throughout her clinical course, neutrophil engraftment was maintained. However, despite an elevated reticulocyte count, she remained dependent on red blood cell and platelet transfusions until her death.
Case 2 (Figure 1B): A 35-year-old man who underwent an AlloSCT for classic Hodgkin lymphoma developed a post-transplant lymphoid neoplasm (PTLD) originating from recipient T cells. He had a refractory clinical course despite chemotherapy and discontinuation of immunosuppressants. Therefore, CBT was chosen as the next treatment option. He was diagnosed with acute GVHD on the skin and treated with oral corticosteroids and tacrolimus, achieving remission. However, discontinuing immunosuppressants to treat PTLD led to worsening erythema and the onset of diarrhea, prompting the initiation of corticosteroid and ruxolitinib at 10 mg/day. Ruxolitinib was effective and allowed for the tapering of corticosteroids.
For CBT, the conditioning regimen consisted of fludarabine (150 mg/m2), melphalan (80 mg/m2), and total body irradiation (8Gy). GVHD prophylaxis included tacrolimus and MMF. The patient developed erythema due to acute GVHD, which appeared rapidly and was closely associated with the tapering and discontinuation of immunosuppressants. Therefore, we continued treatment for acute GVHD with ruxolitinib. Due to concerns regarding hematologic toxicity, the ruxolitinib dose was reduced to 5 mg/day. The cord blood unit contained a nuclear cell count of 3.2 × 107/kg (CD34-positive cells: 0.66 × 105/kg) and was HLA-matched at four out of six antigens and four out of six alleles in both the graft-versus-host and host-versus-graft directions. No donor-specific antibody was detected. On day 8, the patient developed fever, pulmonary edema, and erythema, leading to a diagnosis of pre-engraftment immune reaction (PIR), which resolved with corticosteroids. Neutrophil engraftment was achieved on day 21. On day 22, we stopped ruxolitinib due to the resolution of GVHD symptoms and PIR. STR analysis of peripheral blood on day 30 confirmed complete donor chimerism in both granulocytes and T cells. However, on day 37, bone marrow examination revealed abnormal lymphocytes, and STR analysis showed a mixed chimera on T cells, indicating PTLD relapse. The patient died on day 50 due to PTLD relapse. During his clinical course, neutrophil engraftment was preserved; however, he remained dependent on red blood cells and platelet transfusions until he died.
Discussion
We report two cases of successful neutrophil engraftment following CBT with concurrent ruxolitinib administration. Ruxolitinib was initially approved as a treatment for myelofibrosis and demonstrated effectiveness against symptoms such as splenomegaly and other clinical manifestations4,5. Additionally, ruxolitinib has proven effective for steroid-refractory acute and chronic GVHD6,7. Therefore, ruxolitinib is expected to benefit patients undergoing AlloSCT with myelofibrosis by reducing splenomegaly and improving engraftment potential9, as well as those needing a novel strategy for GVHD prophylaxis. However, its use in AlloSCT raises concerns regarding engraftment due to its hematopoietic effects. In placebo-controlled trials for patients with myelofibrosis, grade 3 or 4 anemia, thrombocytopenia, and neutropenia occurred more frequently with ruxolitinib than with the placebo5. Similarly, higher rates of cytopenia were observed in patients with acute GVHD6 and chronic GVHD, compared with those in the control arms (best available therapy arms)7.
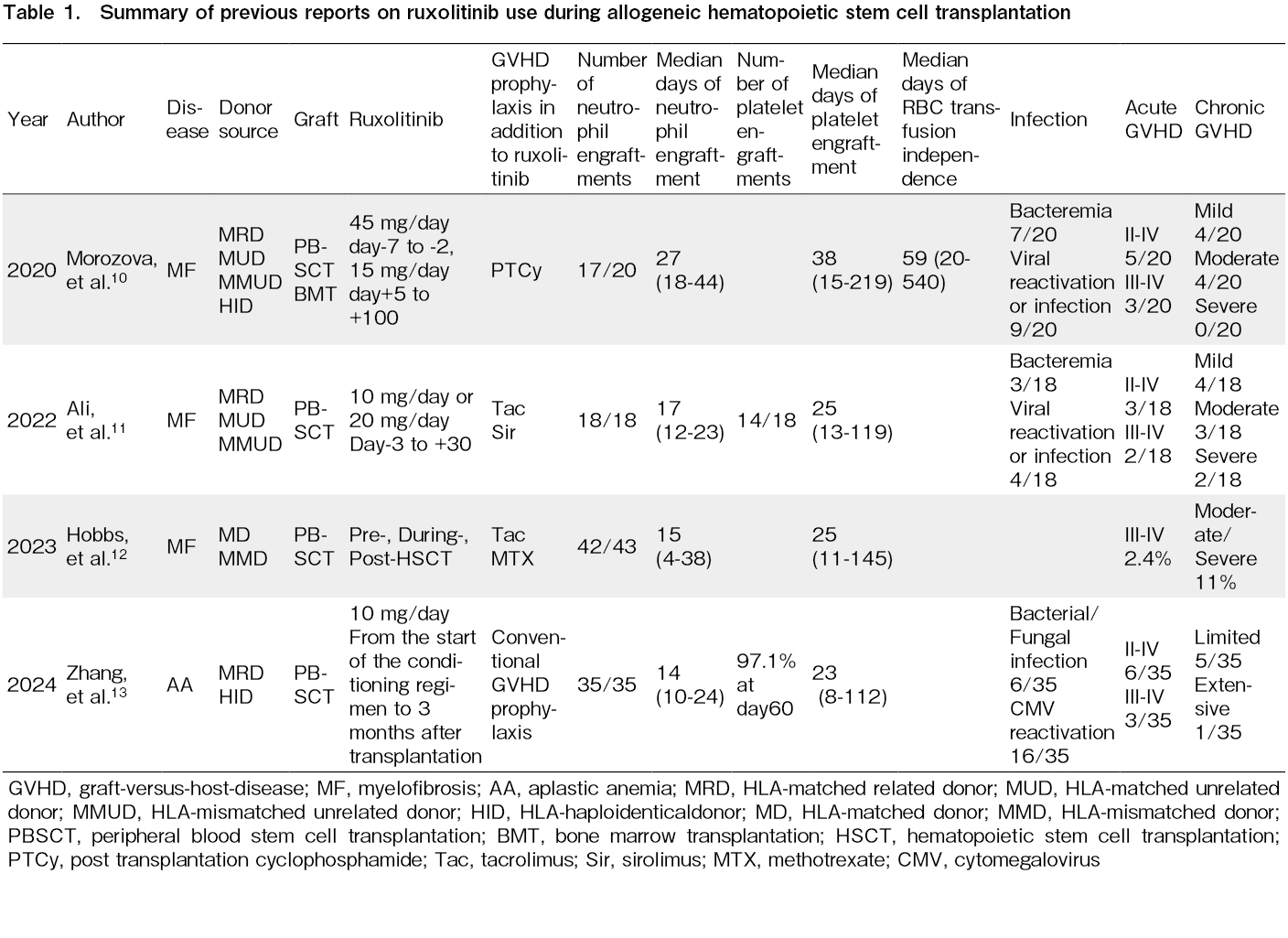
Despite these concerns, some reports have suggested successful engraftment during ongoing ruxolitinib therapy in AlloSCT using bone-marrow or peripheral-blood stem-cell grafts (Table 1). Morozova et al., Ali et al., and Hobbs et al. reported the use of ruxolitinib before and after engraftment in patients with myelofibrosis10–12. Zhang et al. reported a retrospective case series of patients with aplastic anemia who received ruxolitinib during PBSCT13. In that report, two patients were given cord blood on day 4 for poor graft quality while continuing ruxolitinib (10 mg/day). These patients received both PBSCT and CBT, a fundamentally different approach from the single-unit CBT described in our report.
Across these four studies, acceptable neutrophil and platelet engraftment was observed, with no apparent increase in infections or acute/chronic GVHD, compared to their prior experience. While engraftment outcomes were generally favorable, dose modification was sometimes required. Morozova et al. reported a 55% incidence of severe poor graft function (cytopenia with full donor chimerism), necessitating dose reduction or discontinuation10. Zhang et al. noted severe poor graft function in 2 of 35 patients, although the incidence was not higher than that in historical controls13.
In this report, we describe two cases of successful neutrophil engraftment while administering ruxolitinib in single-unit CBT, which has fewer hematopoietic stem and progenitor cells than PBSCT. These cases may imply a possible role for ruxolitinib in CBT for patients with myelofibrosis or those requiring an alternative GVHD prophylaxis. This study had some limitations. The ruxolitinib dose in this study (5 mg/day) was lower than those in prior studies. This dose was chosen based on concerns regarding hematopoietic toxicity, poor graft function, and its efficacy for GVHD control at the time.
Another limitation is the short observation period in both cases, limiting our ability to assess ruxolitinib's impact on erythrocyte and platelet engraftment, infections, GVHD, and disease relapse. In our cases, erythrocyte and platelet engraftment were not achieved. Besides limited survival, TA-TMA and severe hemorrhagic cystitis in Case 1 likely contributed to significant consumption of erythrocytes and platelets. However, it is noteworthy that the patient's reticulocyte count increased, suggesting marrow recovery. Infection is also an important consideration when using ruxolitinib in this setting. In Case 1, severe hemorrhagic cystitis and BK viremia occurred. Although earlier studies reported no increase in infections, compared with standard approaches10,11,13, further experience is particularly needed in the CBT setting.
In addition, our report could not draw conclusions regarding disease relapse. Previous studies suggest a low relapse rate in patients with myelofibrosis10, and some preclinical data indicate that ruxolitinib may treat GVHD while preserving the graft-versus-leukemia effect14. These findings raise expectations for the use of ruxolitinib in the CBT setting. However, Case 2 experienced disease relapse, and the favorable outcomes reported by Morozova et al. may partly reflect the effects of ruxolitinib in myelofibrosis. Therefore, the impact of ruxolitinib on relapse, especially in hematologic malignancies other than myelofibrosis, remains unclear and warrants further clinical investigation.
A recent study showed that PIR is an IL-6-driven syndrome15. IL-6 activates JAK/STAT pathway; hence, ruxolitinib is expected to attenuate PIR. However, a retrospective study demonstrated that a mild PIR following CBT is associated with a lower risk of relapse16. Taken together, the administration of ruxolitinib during the period following CBT until engraftment may attenuate the GVL effect. Our patient still developed steroid-requiring PIR despite continuous ruxiolitinib therapy. Murine data indicate GVL retention, and myelofibrosis reports low relapse10,14; although these findings are encouraging, but larger case series are needed to clarify the impact of ruxolitinib on PIR and relapse.
Despite these limitations, the prompt neutrophil engraftment achieved in this high-risk setting of post-transplant complication following relapses after prior allogeneic transplantation offers encouraging evidence and underpins the rationale for further studies on this strategy. To our knowledge, this is the first report of successful neutrophil engraftment in patients receiving CBT while administering ruxolitinib. Our findings suggest that it may become a promising drug for myelofibrosis patients receiving CBT or for patients who need an alternative GVHD prophylaxis protocol. This report provides valuable insights into extending the therapeutic application of ruxolitinib to CBT recipients, supporting future investigations. While neutrophil engraftment appears satisfactory, the effects on red blood cell and platelet recovery, infections, GVHD, and relapse remain unclear, underscoring the need for further evaluation.
Acknowledgments
The authors thank Editage (
Author Contributions
TN, TK, and KM contributed to the conception and design of the patient treatment. All authors participated in the patient's treatment. TN and TK wrote the first draft. All authors reviewed, revised, and approved the final manuscript.
Conflicts of Interest
YM has received honoraria from Novartis Pharma KK. KM is one of the Editors of Blood Cell Therapy. He was not involved in the editorial evaluation or decision to accept this article for publication. Disclosure forms provided by the authors are available on the web site.
Acknowledgments
The authors thank Editage (
Informed Consent
All patients signed informed consent regarding publishing their data.
References
1.Ward AC, Touw I, Yoshimura A. The Jak-Stat pathway in normal and perturbed hematopoiesis. Blood. 2000; 95: 19-29.
2.Verstovsek S, Mesa RA, Livingston RA, Hu W, Mascarenhas J. Ten years of treatment with ruxolitinib for myelofibrosis: a review of safety. J Hematol Oncol. 2023; 16: 82.
3.Penack O, Marchetti M, Aljurf M, Arat M, Bonifazi F, Duarte RF, et al. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2024; 11: e147-59.
4.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012; 366: 787-98.
5.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012; 366: 799-807.
6.Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-Versus-Host Disease. N Engl J Med. 2020; 382: 1800-10.
7.Zeiser R, Polverelli N, Ram R, Hashmi SK, Chakraverty R, Middeke JM, et al. Ruxolitinib for Glucocorticoid-Refractory Chronic Graft-Versus-Host Disease. N Engl J Med. 2021; 385: 228-38.
8.De Togni E, Cole O, Abboud R. Janus kinase inhibition in the treatment and prevention of graft-versus-host disease. Front Immunol. 2024; 15: 1304065.
9.Kröger NM, Deeg JH, Olavarria E, Niederwieser D, Bacigalupo A, Barbui T, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia. 2015; 29: 2126-33.
10.Morozova EV, Barabanshikova MV, Moiseev IS, Shakirova AI, Barhatov IM, Ushal IE, et al. A Prospective Pilot Study of Graft-versus-Host Disease Prophylaxis with Post-Transplantation Cyclophosphamide and Ruxolitinib in Patients with Myelofibrosis. Acta Haematol. 2021; 144: 158-65.
11.Ali H, Tsai NC, Synold T, Mokhtari S, Tsia W, Palmer J, et al. Peritransplantation ruxolitinib administration is safe and effective in patients with myelofibrosis: a pilot open-label study. Blood Adv. 2022; 6: 1444-53.
12.Hobbs GS, Kim HT, O'Connor S, Schroeder MA, Tamari R, Wall SA. Updated Findings of a Phase II Study of Ruxolitinib Pre-, during- and post-Hematopoietic Stem Cell Transplantation for Patients with Primary or Secondary Myelofibrosis. Blood. 2023; 142 (Suppl 1): 2103.
13.Zhang X, Zhao X, Chen S, Hao M, Zhang L, Gong M, et al. Addition of ruxolitinib to standard graft-versus-host disease prophylaxis for allogeneic stem cell transplantation in aplastic anemia patients. Bone Marrow Transplant. 2024; 59: 997-1005.
14.Schroeder MA, Choi J, Staser K, DiPersio JF. The role of Janus kinase signaling in graft-versus-host disease and graft versus leukemia. Biol Blood Marrow Transplant. 2018; 24: 1125-34.
15.Jin L, Sun Z, Liu H, Zhu X, Zhou Y, Fu B, et al. Inflammatory monocytes promote pre-engraftment syndrome and tocilizumab can therapeutically limit pathology in patients. Nat Commun. 2021; 12: 4137.
16.Yamamoto H, Uchida N, Yuasa M, Kageyama K, Kaji D, Mitsuki T, et al. Pre-Engraftment Immune Reactions Has Unique Graft-Versus-Leukemia Effects After Single Cord Blood Transplantation. Blood. 2019; 134 (Suppl 1): 2041.
Search
News