Volume 8 (2025) Issue 4 No.7 Pages 290-300
Abstract
Background: Viral reactivations frequently complicate unrelated donor (UD) and haploidentical (HI) stem cell transplants (SCTs). We analyzed incidence, risk factors, and outcomes of viral reactivations in alternative donor transplants at our center.
Methods: This retrospective study included all cases of UD or HI transplants performed between January 1, 2009 and February 29, 2020. We identified 196 viral reactivations in 87 patients in this cohort.
Results: The incidence of cytomegalovirus (CMV), Epstein-Barr virus (EBV), and adenovirus (ADV) reactivation was 80.5, 36.7, and 26%, respectively. CMV colitis was observed in six patients (8.5%). Acute graft-versus-host disease (GVHD) was a significant risk factor for CMV reactivation. Four patients (5%) developed ADV disease requiring treatment with cidofovir. Anti-thymocyte globulin use and acute GVHD were significant risk factors for ADV reactivation. Mild hemorrhagic cystitis due to BK virus was observed in 32% patients. However, significant cystitis requiring intervention was seen in only four patients. Two patients developed EBV-related lymphoproliferative disorder and one patient experienced human herpes virus 6-related graft failure. Viral reactivations had no impact on overall survival.
Conclusions: The incidence of CMV reactivation in alternative donor HSCTs is high (80%) in the Indian population. Acute GVHD is a significant risk factor for CMV, ADV, or EBV reactivation. The rates of ADV, BK virus, and EBV reactivation requiring intervention are low. Better prophylactic strategies are required to prevent CMV reactivation in alternative donor transplants.
Introduction
Allogeneic hematopoietic stem cell transplants (HSCTs) are a curative option for high-risk hematologic malignancies. The numbers of alternative donor transplants [unrelated donor transplants (UDTs) and haploidentical transplants (HITs)] have increased in recent years because of improved graft-versus-host disease (GVHD) prophylaxis and comparable outcomes with matched-sibling HSCTs1,2. However, these transplants are limited by an increased incidence of GVHD, delayed immune reconstitution, and prolonged immunosuppression, leading to secondary infections3–5. Viral reactivations pose a major threat in the early post-transplant phase as they may lead to organ involvement, increase the risk of developing GVHD, and cause poor graft function6. Data on the incidence and outcomes of viral reactivations in alternate donor transplants are limited, particularly in South Asian countries. We analyzed the incidence, risk factors, and outcomes of viral reactivations in alternative donor transplants in our HSCT unit to determine the exact burden of viral infections, their impact on survival in this subset of patients, and the utility of viral monitoring in these patients.
Materials and Methods
This was a retrospective study of viral reactivation profiles of all alternative donor HSCT recipients in a tertiary cancer care center in India between January 1, 2009 and February 29, 2020.
Transplant procedure
The conditioning regimen used was either cyclophosphamide (60 mg/kg × 2 days) along with myeloablative total body irradiation (> 8 Gy) or fludarabine-based reduced-intensity chemotherapy. Reduced intensity regimens used were fludarabine (150 mg/m2) in combination with either treosulfan (30-42 g/m2) or melphalan (140 mg/m2) or TBI (4 Gy to 8 Gy) or busulfan (9.6-12.8 mg/kg IV). All patients received T-cell replete grafts. Rabbit anti-thymocyte globulin (ATG) (2.5 mg/kg × 1 or 2 days) or alemtuzumab (Campath) was used in UDTs. Calcineurin inhibitors (CNIs), along with methotrexate, were used as prophylaxis for GVHD in UDTs. Post-transplant cyclophosphamide (50 mg/kg on days +3 and +4) along with CNIs and mycophenolate mofetil was used for HITs. Antifungal prophylaxis with posaconazole or voriconazole was administered to all patients. Acute GVHD was treated with methyl prednisolone (2 mg/kg) or an equivalent, and etanercept was the preferred second-line agent in steroid-refractory cases.
Virus monitoring
Cytomegalovirus (CMV) was monitored twice weekly from the start of conditioning until the patient was on immunosuppressants. After 2011, adenovirus (ADV), BK virus, and Epstein-Barr virus (EBV) were monitored weekly after engraftment until day 100. Virus monitoring was conducted using reverse transcription quantitative PCR (RT-qPCR) in peripheral blood for CMV and EBV and in peripheral blood and urine for ADV and BK virus. Stool was tested for CMV (quantitative) and ADV (qualitative) when clinically indicated. Until 2018, CMV reactivation was defined as the occurrence of two consecutive quantitative PCR readings > 500 copies/mL. Due to the observed high incidence of CMV infection in the haploidentical transplant (HIT) cohort, this threshold was revised post-2018 to a more sensitive criterion―two consecutive CMV PCR readings > 150 copies/mL. Resolution of CMV reactivation was defined as two consecutive undetectable CMV PCR results following the initiation of pre-emptive antiviral therapy. If CMV DNAemia reappeared above the defined threshold after achieving undetectable levels, it was classified as a second episode of CMV reactivation in the same patient. Colonoscopy with biopsy was performed when clinically indicated and technically feasible. A diagnosis of CMV colitis was established by histopathological evidence of CMV inclusion bodies or positive CMV PCR on colonic mucosal biopsies.
Parvovirus and human herpes virus-6 (HHV-6) were monitored when clinically indicated. ADV, EBV, BK virus, or parvovirus reactivation was defined as > 150 copies/mL in blood/urine/stool. Monitoring was continued until 3 weeks after discontinuation of immunosuppressants and restarted at the onset of acute or chronic GVHD or the use of systemic steroids. Hepatitis B virus (HBV) and hepatitis C virus (HCV) serology were tested pre-transplantation in all patients. Hepatitis serology and viral load were tested post-transplantation in case the patient developed transaminitis.
Viral prophylaxis and treatment
All patients received oral acyclovir (400 mg three times a day) prophylaxis against herpes and varicella virus from the start of conditioning until 1-year post-transplantation or for an extended duration in the case of herpes reactivation. Intravenous (i.v.) ganciclovir (5 mg/kg/dose twice a day, reduced to 2.5 mg/kg/dose if creatinine clearance < 60 mL/min) was used as a first-line pre-emptive agent for CMV reactivation. In case of grade 3 cytopenia precluding the use of ganciclovir or ganciclovir failure, artesunate or leflunomide was used. Foscarnet and cidofovir were used rarely because of unavailability. Asymptomatic adenoviremia and asymptomatic adenoviral shedding in urine were monitored, and treatment was initiated when adenoviral disease was probable or demonstrated. Cidofovir (5 mg/kg i.v.) was administered (whenever it could be procured) weekly for a maximum of 4 weeks, followed by every 2 weeks until resolution of adenoviral disease. Hemorrhagic cystitis due to BK virus reactivation was managed with supportive treatment including hyperhydration, analgesia, tapering of immunosuppression, and intravesical granulocyte colony-stimulating factor. Post-transplant lymphoproliferative disorder (PTLD) resulting from EBV reactivation was managed with weekly rituximab (375 mg/m2). Patients who had serologic evidence of past HBV infection [defined as positivity for anti-Hepatits B core (HBc) immunoglobin (Ig)M or IgG, or positivity for anti-Hepatitis B surface (HBs) antibodies in the absence of a history of vaccination] were started on prophylaxis with oral entecavir 0.5 mg or lamivudine 100 mg once daily pre-transplantation. The choice of entecavir versus lamivudine was based on HBV DNA (with lamivudine being administered to HBV DNA-negative patients and entecavir in case of HBV DNA-positivity with any titer). Entecavir or lamivudine for HBV reactivation was initiated in case of transaminitis with new-onset
Viral DNA detection and quantitation
CMV DNA was isolated and identified using a CMV PCR assay used at our center, as described previously8. ADV, EBV, parvovirus, HHV-6, and BK virus DNA was extracted using Roche High Pure Viral RNA kit (Roche Diagnostics, Mannheim, Germany) and quantified using RT-qPCR in a CFX 96 thermal cycler (Bio-Rad Laboratories, Hercules, CA, USA) or a Qiagen Rotor-Gene Q5 real-time PCR system (QIAGEN GmbH, Hilden, Germany), as per the manufacturers’ instructions. The limit of detection was 10 to 5 × 109 copies/mL for ADV, 50 to 5 × 1010 copies/mL for BK virus and parvovirus, and 25 to 2 × 109 IU/μL for EBV. HHV-6 reactivation was diagnosed using qualitative PCR. Anti-HBs and Anti-HBc IgM/total were tested using a chemiluminescent microparticle immunoassay and enzyme-linked fluorescence assay, respectively, whereas HBV DNA was quantified using RT-qPCR, with a detection limit of 50 to 6.38 × 108 copies/mL.
Statistical analysis
All data were entered in a Microsoft excel spreadsheet and statistically analyzed using SPSS version 21.0. The primary endpoint was the incidence of viral reactivation. The secondary endpoint was overall survival. Survival analysis was conducted using the Kaplan-Meier method, and survival rates were compared using the log-rank test. Clinical, transplant, and viral characteristics are represented as median with range, and proportions are expressed as percentages with 95% confidence intervals (CIs) where apt. The odds ratio (OR) was used to assess the strength of the association between various risk factors and viral reactivations. Institutional Ethics committee (IEC III,
Results
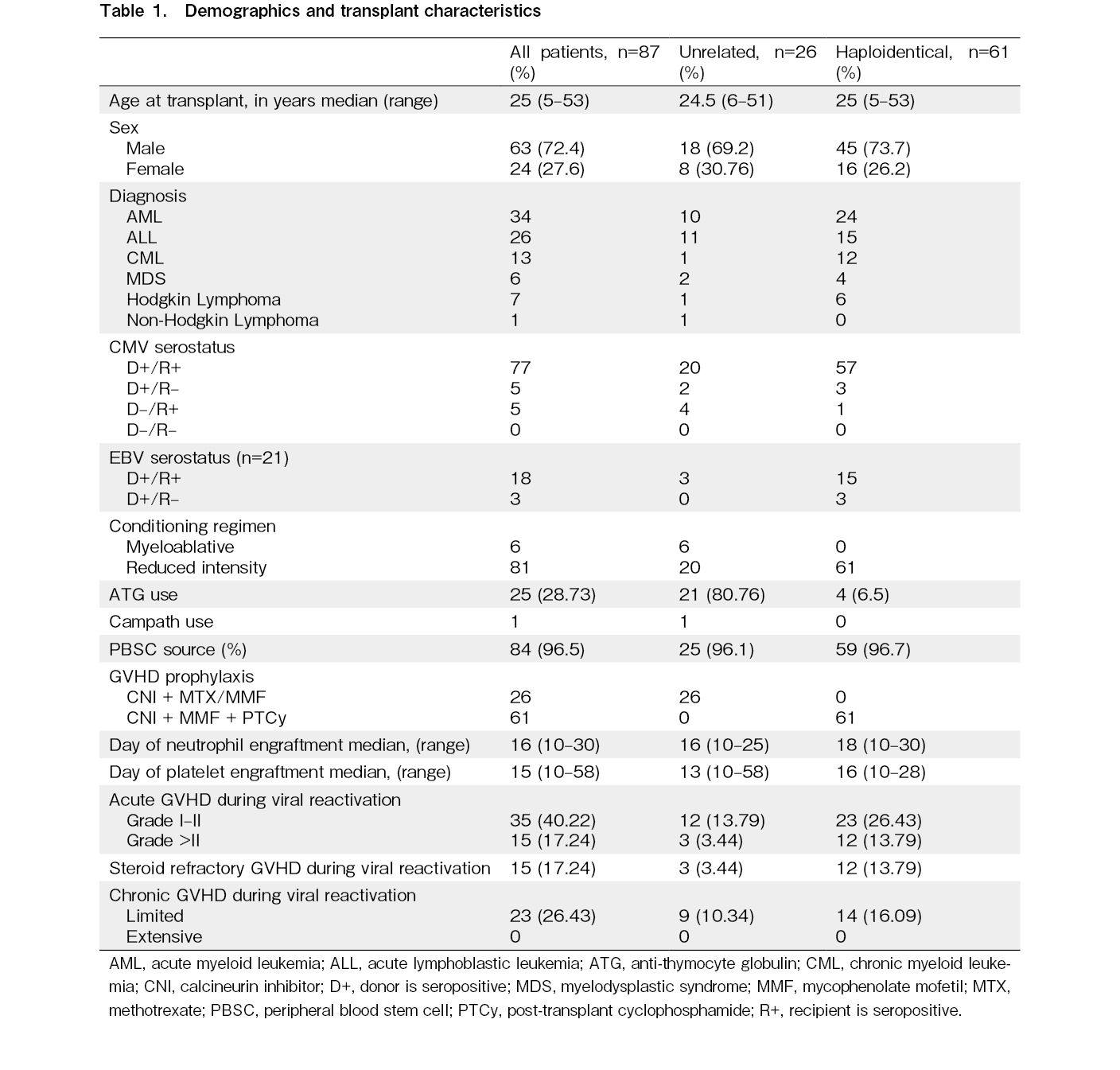
In total, 703 transplants were conducted between January 1, 2009 and 29 February 29, 2020. Of these, 334 were allogeneic transplants, including 87 alternative donor transplants, encompassing 26 UDTs and 61 HITs. Among the 26 UDTs, 21 were fully human leukocyte antigen-matched transplants, three were mismatched (9/10) unrelated transplants, and two were 5/6 cord transplants. Peripheral blood stem cells were the predominant source (n=83; 96%), whereas bone marrow was the source in two patients and cord blood in another two patients. Fludarabine-based reduced-intensity conditioning was provided to 93% of patients (81/87), and cyclophosphamide and myeloablative total body irradiation conditioning to six patients. The baseline characteristics of the patients, their diseases, and transplant characteristics are provided in Table 1.
CMV reactivation
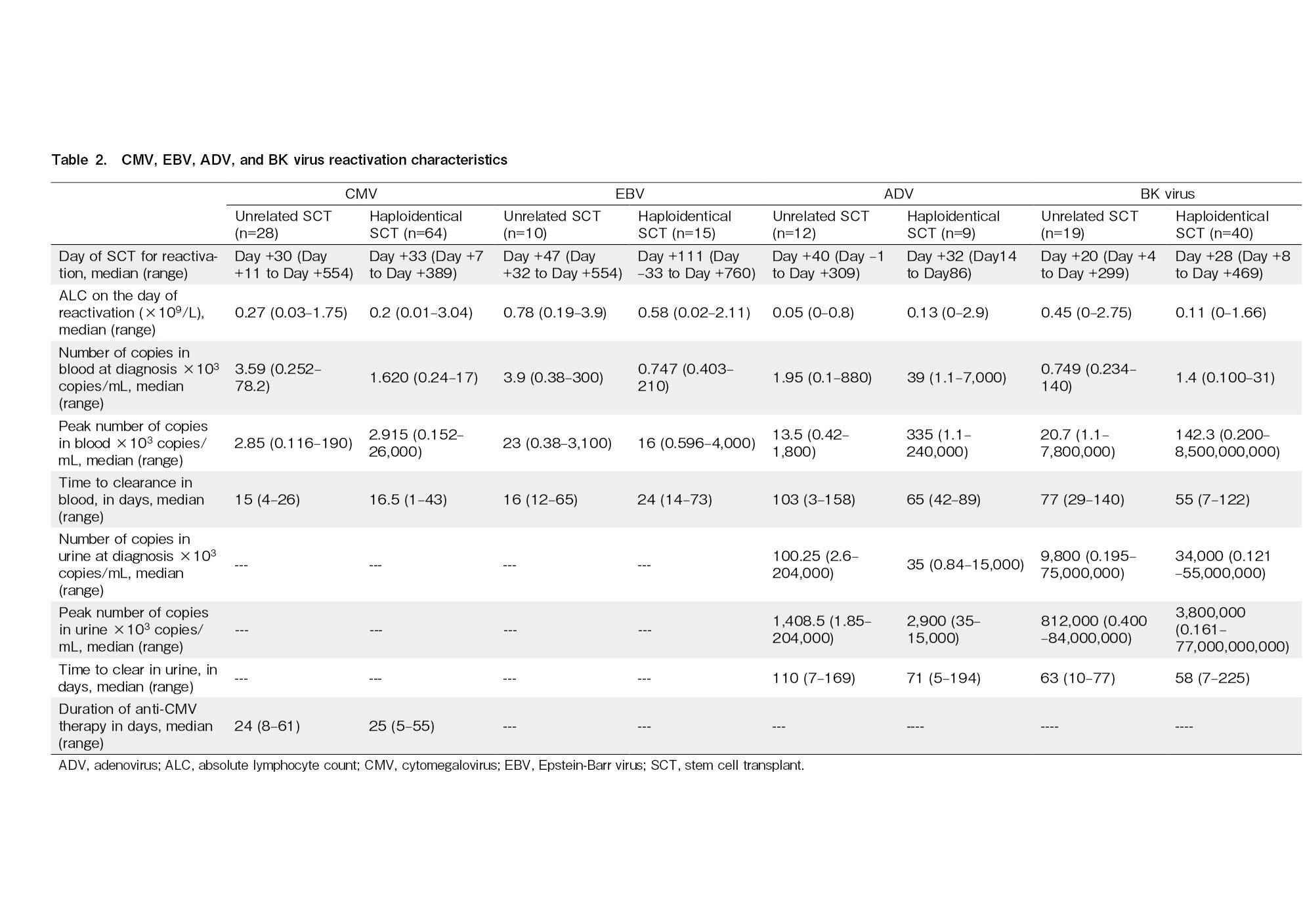
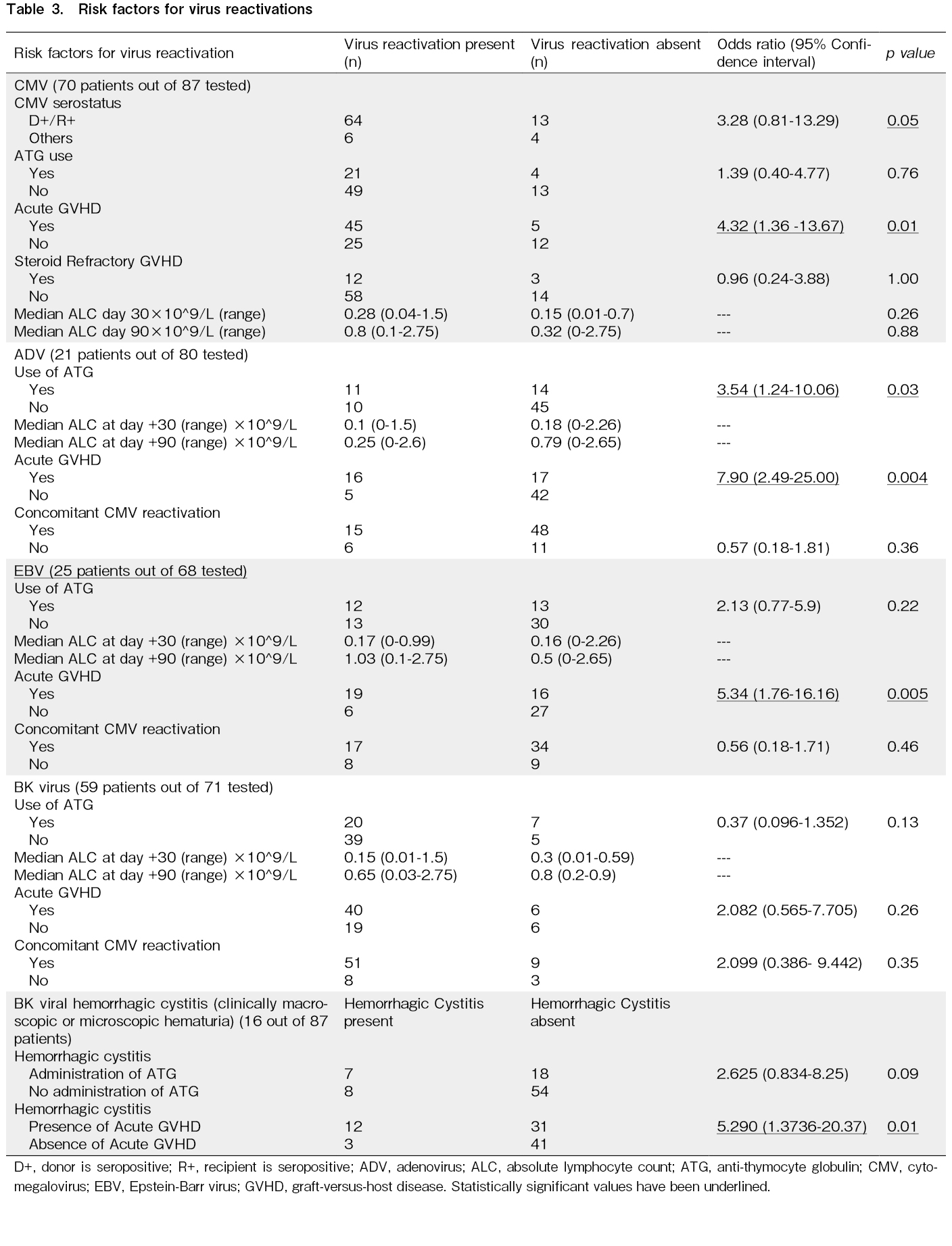
There were 92 episodes of CMV reactivation in 70 of the 87 transplants in total (incidence, 80.46%; 95% CI, 70%-88%). We identified 28 episodes in 21 UDTs, all of which were treated pre-emptively. Ganciclovir was used as the first-line agent for pre-emptive treatment in 20 (71%) episodes, artesunate in six episodes, and foscarnet and cidofovir in one episode each. Two patients with UDTs developed CMV colitis. Among HITs, we found 64 episodes of CMV reactivation in 49 patients. Ganciclovir was used as the first line in 39 episodes (61%), artesunate in four episodes (6%), foscarnet in two (3%) episodes, and leflunomide in 19 episodes (30%). CMV colitis was observed in four HIT patients, of whom three developed colitis within 100 days. Patients who had CMV reactivation were four times more likely to have acute GVHD during the reactivation period (OR, 4.32; 95% CI, 1.361-13.67; p=0.013). The details of CMV reactivation and risk factors according to multivariate analysis are shown in Table 2 and 3, respectively.
ADV reactivation
Twenty-one of eighty patients who were tested weekly for viral reactivations developed ADV reactivation (incidence, 26.25%; 95% CI, 17%-37%), including 12 of 25 UDT (48%) and 9 of 55 (16%) HIT patients. Four patients (5% of those tested) developed ADV disease and were treated with cidofovir: two had ADV colitis, one had ADV cystitis, and one had ADV colitis along with central nervous system involvement. Two of these four patients died with ADV disease. Patients who had ADV reactivation were thrice as likely to have received ATG compared to those who did not have reactivation (OR, 3.54; 95% CI, 1.24-10.06; p=0.03). Moreover, these patients were eight times more likely to have acute GVHD during the ADV reactivation episode (OR, 7.90; 95% CI, 2.49-25; p=0.0004). The characteristics of adenoviremia and adenoviruria are presented in Table 2, and the risk factors are shown in Table 3.
EBV reactivation
EBV reactivation occurred in 25 of 68 patients tested (incidence, 36.7%; 95% CI, 25%-49%), including 10 (50%) of 20 UDT and 15 (31%) of 48 HIT patients. The characteristics and risk factors are presented in Tables 2 and 3, respectively. Two patients developed PTLD requiring therapy with weekly rituximab, one of whom died due to uncontrolled PTLD. The remainder of the patients were asymptomatic and did not require any specific intervention, and EBV copies spontaneously declined after tapering or withdrawal of immunosuppressants.
BK virus reactivation
BK virus reactivation was observed in 59 of 71 patients tested (incidence, 83.1%; 95% CI, 72%-90%), including 19 of 26 (73%) UDT and 40 of 45 (88%) HIT patients. BK virus reactivation characteristics in blood and urine are presented in Table 2. Grade I/II hemorrhagic cystitis was observed in 7 recipients and grade III/IV in 8 recipients, as per the Droller grading system9. The remainder were asymptomatic with no evidence of microscopic or gross hematuria. All patients were treated with intravenous hydration. None received cidofovir for BK virus reactivation. Foley catheterization and bladder irrigation with cold saline were performed in three patients, and intravesical granulocyte colony-stimulating factor was administered to one patient. Presence of acute GVHD was associated with higher rate of hemorrhagic cystitis (OR, 5.29; 95% CI, 1.37-20.37; p=0.015) (Table 3).
Parvovirus reactivation
Two of the twelve patients tested developed parvovirus reactivation. One was a 9-year-old child with symptomatic anemia and a drop in hemoglobin from 8.6 g% to 4.7 g% over 15 days. The highest parvovirus load in peripheral blood was 3.0 × 109 copies/mL on day +217 after transplantation. Hemoglobin improved to 7.4 g% within 2 weeks of initiation of i.v. immunoglobulin therapy. The second patient had asymptomatic parvo-viremia (3.9 × 103 copies/mL), which resolved without any intervention.
HHV-6 reactivation
One of the fifteen patients tested had HHV-6 reactivation as indicated by qPCR on day +40. The patient died on day 57 after transplantation owing to a fungal brain abscess without clearance of HHV-6.
HBV reactivation
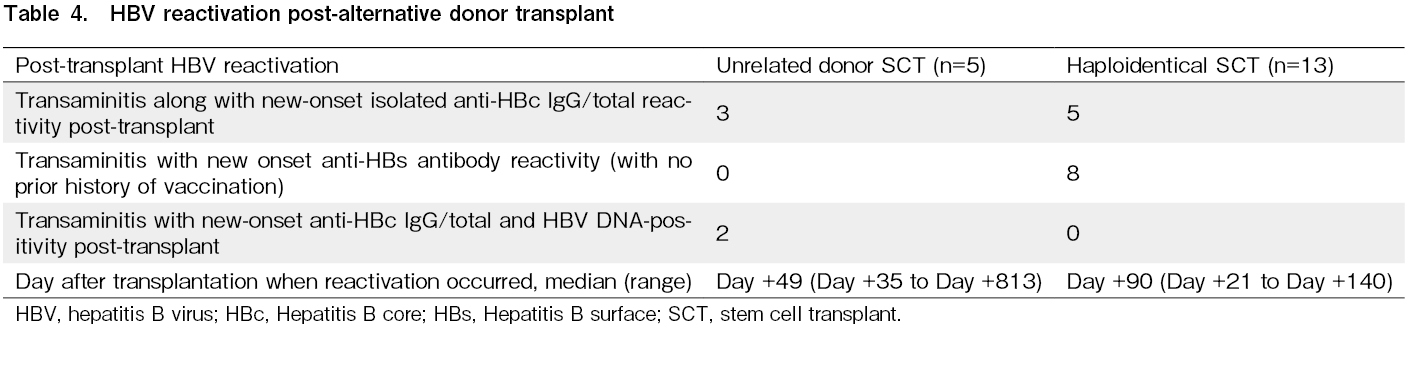
Thirty-one patients (35%) were started on lamivudine or entecavir prophylaxis because of positive HBV serology pre-transplantation. In total, 18 patients (20.68%; 95% CI, 12.75%-30.71%) underwent UDT or HIT and developed post-transplant HBV reactivation (new-onset positive serology with transaminitis), of whom eight developed reactivation within 100 days (Table 4). Of these 18 patients, only two tested HBV DNA-positive in peripheral blood. There were no cases of co-existing liver GVHD/chronic GVHD. Entecavir was started in eight and lamivudine in nine of these patients for post-transplant HBV reactivation and continued for 2 years post-transplantation. HCV reactivation was not observed in this cohort.
The number of patients with Parvovirus reactivations, HHV-6 reactivations and hepatitis B DNA positivity were too low to show statistically robust correlation with risk factors and hence these were not analyzed.
Outcomes
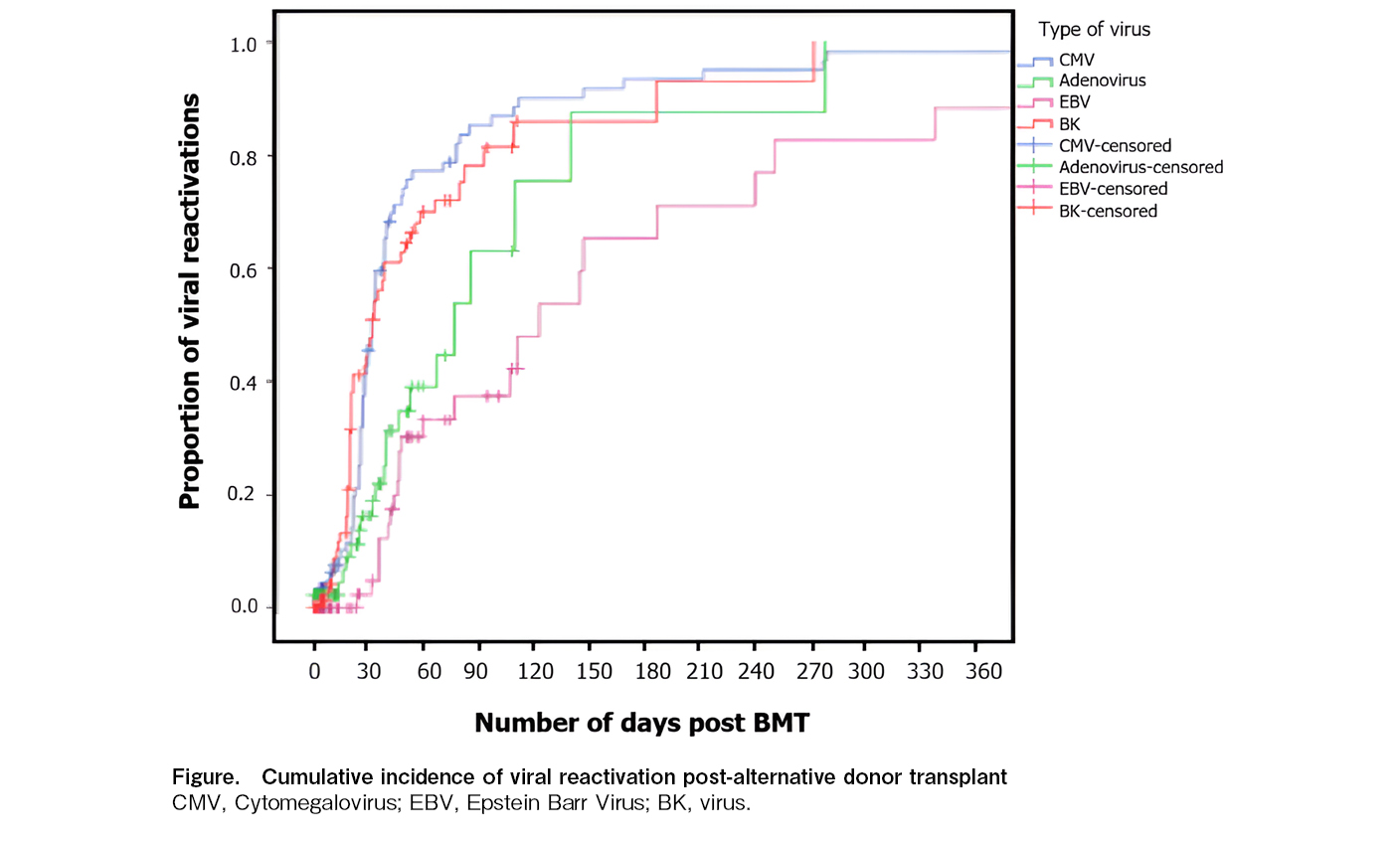
We identified a total of 196 viral reactivations (excluding herpes simplex and herpes zoster) in 87 patients who received alternative donor SCTs. The median overall survival for the entire cohort was 52.14 months (95% CI, 43.8-60.4 months). Despite the high incidence of CMV reactivation (80%), CMV end-organ involvement was observed in only four patients, indicating that pre-emptive treatment was largely successful, and there was no direct mortality because of CMV disease. Two patients and one patient died because of ADV disease and EBV-related PTLD, respectively. The cumulative incidence of major viral infections is shown in Figure. Viral reactivations had no statistically significant effect on overall survival in this cohort.
Discussion
We systematically monitored UDT and HIT patients between 2009 and 2020 for viral reactivations. Data on viral reactivations post alternate donor transplants from South Asian countries is sparse. The incidence of CMV reactivation was in our very high, around 80%, in both HITs and UDTs. This is much higher compared to incidence in western countries which ranges from 35-50%10–12. The CMV reactivation rates are much higher in other Asian countries- and range from 55-60%13. The high reactivation rate in Indian patients is likely because of a high rate of donor/recipient (D+/R+) CMV seropositivity (88%) in our ethnic group, as described in the literature10,11. Several other studies have reported a high risk of reactivation of 30%-71% in UDTs and HITs12,14,15 because of delayed immune reconstitution and ex vivo T-cell depletion. In a study by George et al., fludarabine-based reduced-intensity conditioning was associated with a high risk of CMV reactivation16,17. Our cohort predominantly received fludarabine-based reduced-intensity conditioning (n=81, 93.10%). The majority of reactivations occurred in the immediate post-engraftment phase, and acute GVHD occurred in 50 patients (57.47%), 90% of whom developed CMV reactivation. Steroid-refractory acute GVHD was observed in 15 of 50 (30%) patients, of whom 12 (80%) developed CMV reactivation. This is because steroid and immunosuppressive therapies further increase the risk and is concordant with previous studies in which acute GVHD, human leukocyte antigen disparity6,18, showed a higher risk of CMV reactivation. Lymphopenia has been associated higher risk of CMV end organ disease and death in both allogeneic and autologous transplant setting19,20. Despite the high rate of reactivation, CMV end-organ involvement was observed in only 8.6% of patients who experienced reactivation. There was no direct mortality attributed to CMV pneumonitis/hepatitis or enterocolitis. Active qPCR-based CMV surveillance twice a week and prompt initiation of pre-emptive anti-CMV therapy reduced the incidence of life-threatening CMV disease. However, secondary bacterial and fungal infections were a major cause of mortality in this cohort.
As primary ADV infection occurs in childhood, the risk of reactivation is generally increased in pediatric allogenic transplants, with an incidence of 2.5%-21%21,22. The current study cohort comprised a predominantly young-adult population, with a median age of 25 years, and the cumulative incidence of ADV infection was 24.13% (in the entire cohort of 87 alternative donor transplants). Concomitant CMV reactivation was observed in 15 of 21 patients (71.4%). These findings are consistent with findings in several other studies in which alternative donor SCT, acute GVHD, prolonged immunosuppression, and an absolute lymphocyte count < 200/mm3 were significant risk factors for ADV reactivation23,24.
The cumulative incidence of EBV reactivation was 28.73% in our entire cohort. Twelve of twenty-five (48%) patients who received ATG developed EBV-DNAemia. Reduced-intensity conditioning, in vivo/ex vivo T-cell depletion, cord and UDT, and acute grade II to IV GVHD are well-established risk factors for EBV-DNAemia and PTLD25–28. Although the incidence of PTLD reportedly increases following UDT or HIT29,30, we encountered PTLD in only two recipients. This may be because most HIT patients (61/87) received post-transplant cyclophosphamide rather than ATG in our cohort30.
BK virus reactivation was the second most common viral reactivation after CMV reactivation, and the cumulative incidence was 59%, likely because of the high proportion of HITs32. The majority of patients with BK virus reactivation were asymptomatic (61%). According to the literature, routine BK virus monitoring as a screening tool for hemorrhagic cystitis has yielded variable results; therefore, its clinical utility has been questioned33,34. Accordingly, the European conference on infections in leukemia (ECIL)-6 guidelines do not recommend routine BK virus monitoring in allo-HSCT recipients35.
Studies have reported a high HHV-6 incidence of up to 30% in the pediatric population and of 7%-30% in adult transplant recipients, particularly in the early post-transplant phase, because of insufficient virus-specific T-cell immune responses36,37. In our cohort, the incidence of reactivation was low: 1.1% for HHV-6 and 2.2% for parvovirus. This is because routine surveillance was not conducted for these viruses; monitoring was conducted only when clinically indicated. The incidence of HBV reactivation in our cohort was 20%, which is in line with findings by reported by Hammond et al.38. Antiviral therapy for isolated anti-HBs antibody positivity was initiated in recipients without a history of vaccination and with deranged liver enzymes, although data to suggest treatment initiation are sparse in this setting7. The early initiation of antiviral therapy probably limited long-term complications due to HBV reactivation. To the best of our knowledge, the present dataset is one of the largest datasets on viral reactivations in an alternate donor transplant setting in the Indian population. Based on our study, the regular monitoring of ADV, BK virus, and EBV may have limited utility due to the low incidence of symptomatic disease requiring therapeutic intervention. Cytomegalovirus and hepatitis B are the important viruses of concern. This is a notable finding, and resources need to be focused on the detection these viruses, particularly in countries associated with financial constraints, such as India and other South Asian countries, rather than on the regular monitoring of all infectious viruses. This study was limited by its retrospective nature, heterogeneity of the study population in terms of the diagnosis of underlying hematological malignancies, and the long study period, which may have resulted in changes in the standards of monitoring and treatment of viral reactivations during the study period. We also acknowledge that the age group of the cohort varied from 5 years to 53 years, and the reactivation rates will also be affected by the age group of transplanted patients. Nevertheless, our data provide useful insights into incidence and burden of viral reactivation, viral reactivation patterns in alternative donor transplants in the south Asian context.
In conclusion, the incidence of CMV reactivation in alternative donor HSCTs is high (80%) in the Indian population, and acute GVHD is a significant risk factor for CMV, ADV, or EBV reactivation. ATG use is a significant risk factor for ADV reactivation. The rates of ADV, BK virus, and EBV reactivation requiring intervention are low. Better prophylactic strategies are required to prevent CMV reactivation in alternative donor transplants.
Author Contributions
AG and VM: data collection, analysis, and writing of the draft; SP, SM, AC, NJ, AB, LR, and BB: patient care and monitoring; VB, SB, RT, and SKumar: laboratory work and viral PCRs; SKanan: statistical inputs; GB: reviewing and editing the manuscript; NK: concept and overall study mentorship; all authors: input on manuscript and editing. AK and VM contributed equally to this work.
Conflicts of Interest
The author declares no conflict of interest. Disclosure form provided by the author are available on the website. NK is one of the Editors of Blood Cell Therapy. He was not involved in the editorial evaluation or decision to accept this article for publication.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
1.Nagler A, Labopin M, Houhou M, Aljurf M, Mousavi A, Hamladji RM, et al. Outcome of haploidentical versus matched sibling donors in hematopoietic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. J Hematol Oncol. 2021; 14: 53.
2.Saber W, Opie S, Rizzo JD, Zhang MJ, Horowitz MM, Schriber J. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood. 2012; 119: 3908-16.
3.Rashidi A, Hamadani M, Zhang MJ, Wang HL, Abdel-Azim H, Aljurf M, et al. Outcomes of haploidentical vs matched sibling transplantation for acute myeloid leukemia in first complete remission. Blood Adv. 2019; 3: 1826-36.
4.Ghosh N, Karmali R, Rocha V, Ahn KW, DiGillio A, Hari PN, et al. Reduced-Intensity Transplantation for Lymphomas Using Haploidentical Related Donors Versus HLA-Matched Sibling Donors: A Center for International Blood and Marrow Transplant Research Analysis. J Clin Oncol. 2016; 34: 3141-9.
5.Ahmed S, Kanakry JA, Ahn KW, Litovich C, Abdel-Azim H, Aljurf M, et al. Lower Graft-versus-Host Disease and Relapse Risk in Post-Transplant Cyclophosphamide-Based Haploidentical versus Matched Sibling Donor Reduced-Intensity Conditioning Transplant for Hodgkin Lymphoma.. Biol Blood Marrow Transplant. 2019; 25: 1859-68.
6.Lin CH, Su YJ, Hsu CY, Wang PN, Teng CJ. Haploidentical allogeneic hematopoietic stem cell transplantation increases the risk of cytomegalovirus infection in adult patients with acute leukemia. Transpl Infect Dis. 2019; 21: e13096.
7.Gupta A, Punatar S, Gawande J, Bagal B, Mathew L, Bhat V, et al. Hepatitis B-related serological events in hematopoietic stem cell transplant patients and efficacy of lamivudine prophylaxis against reactivation. Hematol Oncol. 2016; 34: 140-6.
8.Gokarn A, Toshniwal A, Pathak A, Arora S, Bonda A, Punatar S, et al. Use of Leflunomide for Treatment of Cytomegalovirus Infection in Recipients of Allogeneic Stem Cell Transplant. Biol Blood Marrow Transplant. 2019; 25: 1832-6.
9.Droller MJ, Saral R, Santos G. Prevention of cyclophosphamide-induced hemorrhagic cystitis. Urology. 1982; 20: 256-8.
10.Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004; 103: 2003-8.
11.Vaezi M, Kasaeian A, Souri M, Soufiyan F, Shokri Boosjin A, Setarehdan SA, et al. How Do Donor-Recipient CMV Serostatus and Post-Hematopoietic Stem Cell Transplantation CMV Reactivation Affect Outcomes in Acute Leukemia Patients?. Int J Hematol Oncol Stem Cell Res. 2017; 11: 199-208.
12.Tischer J, Engel N, Fritsch S, Prevalsek D, Hubmann M, Schulz C, et al. Virus infection in HLA-haploidentical hematopoietic stem cell transplantation: incidence in the context of immune recovery in two different transplantation settings. Ann Hematol. 2015; 94: 1677-88.
13.Lin X, Ou Y, Long H, Huang Y, Song C, Lu Z, et al. [Cytomegalovirus infection after haploidentical stem cell transplantation may reduce relapse risk in leukemia]. Zhonghua Nei Ke Za Zhi. 2016; 55: 107-10. (in Chinese)
14.Piñana JL, Martino R, Barba P, Margall N, Roig MC, Valcárel D, et al. Cytomegalovirus infection and disease after reduced intensity conditioning allogeneic stem cell transplantation: single-centre experience. Bone Marrow Transplant. 2010; 45: 534-42.
15.Di Stasi A, Milton DR, Poon LM, Hamdi A, Rondon G, Chen J, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transpl. 2014; 20: 1975-81.
16.George B, Gillroy N, Kerridge I, Gottlieb D, Hertzberg M, Bradstock K, et al. Higher Incidence of CMV Reactivation in CMV Seropositive Recipients Following Fludarabine Based Reduced Intensity Conditioning Transplants – A Retrospective Comparison with Myeloablative Transplants. Transplant Cell Ther. 2009; 15 (Suppl): 103.
17.George B, Kerridge I, Gilroy N, Huang G, Hertzberg M, Gottlieb D, et al. Fludarabine-based reduced intensity conditioning transplants have a higher incidence of cytomegalovirus reactivation compared with myeloablative transplants. Bone Marrow Transplant. 2010; 45: 849-55.
18.Auletta JJ, Ardura MI, Vasu S, Huang Y, Zhao Q, Ruppert AS, et al. Cytomegalovirus Reactivation Does Not Increase Subsequent Risk for Acute Graft-Versus-Host Disease, Malignant Disease Relapse, or Infection Following Allogeneic Hematopoietic Cell Transplantation. Blood. 2016; 128: 3409.
19.Einsele H, Ehninger G, Steidle M, Fischer I, Bihler S, Gerneth F, et al. Lymphocytopenia as an unfavorable prognostic factor in patients with cytomegalovirus infection after bone marrow transplantation. Blood. 1993; 82: 1672-8.
20.de Moura Almeida A, Macedo EM, Mac Donald Bley C, Kerbauy FR, Vidal Campregher P, Odone V, et al. Cytomegalovirus Infection and Lymphopenia Are Associated with Increased Mortality Post Autologous Stem Cell Transplantation. Blood. 2012; 120: 4208.
21.Yilmaz M, Chemaly RF, Han XY, Thall PF, Fox PS, Tarrand JJ, et al. Adenoviral infections in adult allogeneic hematopoietic SCT recipients: a single center experience. Bone Marrow Transplant. 2013; 48: 1218-23.
22.Baldwin A, Kingman H, Darville M, Foot AB, Grier D, Cornish JM, et al. Outcome and clinical course of 100 patients with adenovirus infection following bone marrow transplantation. Bone Marrow Transplant. 2000; 26: 1333-8.
23.Robin M, Marque-Juillet S, Scieux C, Peffault de Latour R, Ferry C, Rocha V, et al. Disseminated adenovirus infections after allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcome. Haematologica. 2007; 92: 1254-7.
24.Hubmann M, Fritsch S, Zoellner AK, Prevalsek D, Engel N, Bücklein V, et al. Occurrence, risk factors and outcome of adenovirus infection in adult recipients of allogeneic hematopoietic stem cell transplantation. J Clin Virol. 2016; 82: 33-40.
25.Gao XN, Lin J, Wang LJ, Li F, Li HH, Wang SH, et al. Risk factors and clinical outcomes of Epstein-Barr virus DNAemia and post-transplant lymphoproliferative disorders after haploidentical and matched-sibling PBSCT in patients with hematologic malignancies. Ann Hematol. 2019; 98: 2163-77.
26.Cohen JM, Cooper N, Chakrabarti S, Thomson K, Samarasinghe S, Cubitt D, et al. EBV-related disease following haematopoietic stem cell transplantation with reduced intensity conditioning. Leuk Lymphoma. 2007; 48: 256-69.
27.Ru Y, Zhang X, Song T, Ding Y, Zhu Z, Fan Y, et al. Epstein-Barr virus reactivation after allogeneic hematopoietic stem cell transplantation: multifactorial impact on transplant outcomes. Bone Marrow Transplant. 2020; 55: 1754-62.
28.Uhlin M, Wikell H, Sundin M, Blennow O, Maeurer M, Ringden O, et al. Risk factors for Epstein-Barr virus-related post-transplant lymphoproliferative disease after allogeneic hematopoietic stem cell transplantation. Haematologica. 2014; 99: 346-52.
29.Brunstenin CG, Weisdorf DJ, DeFor T, Barker JN, Tolar J, van Burik JAH, et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006; 108: 2874-80.
30.Van Besien K, Bachier-Rodriguez L, Satlin M, Brown MA, Gergis U, Guarneri D, et al. Prophylactic rituximab prevents EBV PTLD in haplo-cord transplant recipients at high risk. Leuk Lymphoma. 2019; 60: 1693-96.
31.Kanakry JA, Kasamon YL, Bolaños-Meade J, Borrello IM, Brodsky RA, Fuchs EJ, et al. Absence of post-transplantation lymphoproliferative disorder after allogeneic blood or marrow transplantation using post-transplantation cyclophosphamide as graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2013; 19: 1514-7.
32.Ruggeri A, Roth-Guepin G, Battipaglia G, Mamez AC, Malard F, Gomez A, et al. Incidence and risk factors for hemorrhagic cystitis in unmanipulated haploidentical transplant recipients. Transpl Infect Dis. 2015; 17: 822-30.
33.Ghosh A, Tan TT, Linn YC, Gopalakrishnan S, Goh YT, Hwang W, et al. What We Learned From Plasma BK-Virus Monitoring in Allogeneic Hematopoietic Transplant Recipients. Transplantation. 2016; 100: e17-18.
34.Cesaro S, Tridello G, Pillon M, Calore E, Abate D, Tumino M, et al. A Prospective Study on the Predictive Value of Plasma BK Virus-DNA Load for Hemorrhagic Cystitis in Pediatric Patients After Stem Cell Transplantation. J Pediatric Infect Dis Soc. 2015; 4: 134-42.
35.Cesaro S, Dalianis T, Hanssen Rinaldo C, Koskenvuo M, Pegoraro A, Einsele H, et al. ECIL guidelines for the prevention, diagnosis and treatment of BK polyomavirus-associated haemorrhagic cystitis in haematopoietic stem cell transplant recipients. J Antimicrob Chemother. 2018; 73: 12-21.
36.Perruccio K, Sisinni L, Perez-Martinez A, Valentin J, Capolsini I, Speranza Massei M, et al. High Incidence of Early Human Herpesvirus-6 Infection in Children Undergoing Haploidentical Manipulated Stem Cell Transplantation for Hematologic Malignancies. Biol Blood Marrow Transplant. 2018; 24: 2549-57.
37.Tamaki H, Ikegame K, Yoshihara S, Kaida K, Yoshihara K, Inoue T, et al. Low incidence of HHV-6 reactivation in haploidentical hematopoietic stem cell transplantation with corticosteroid as graft-vs-host disease prophylaxis compared with cord blood transplantation.. Transpl Infect Dis. 2019; 21: e13073.
38.Hammond SP, Borchelt AM, Ukomadu C, Ho VT, Baden LR, Marty FM. Hepatitis B virus reactivation following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009; 15: 1049-59.
Search
News