Volume 8 (2025) Issue 4 No.4 Pages 274-280
Abstract
Epstein-Barr virus (EBV)-associated T-cell lymphoproliferative disease (EBV+ T-cell LPD), including chronic active Epstein-Barr virus (CAEBV) disease, can affect the central nervous system (CNS) in children, albeit rarely reported in adults. We report a case of an adult with EBV+ T-cell LPD and CNS involvement treated with haploidentical stem cell transplantation (haplo-SCT) and post-transplant cyclophosphamide (PTCy). A 59-year-old woman developed a high fever and elevated lactate dehydrogenase (LDH) levels. Based on the clinical course, high serum EBV-DNA levels, and the presence of EBV-infected T cells in her bone marrow, she was diagnosed with EBV+ T-cell LPD. The patient initially responded to cooling therapy and chemotherapy, leading to a marked decrease in EBV-DNA levels. Subsequently, she experienced a relapse in the CNS, as evidenced by elevated EBV-DNA levels in the cerebrospinal fluid. After chemotherapy and whole-brain irradiation, the patient underwent haplo-SCT with PTCy, following reduced-intensity conditioning. The EBV-DNA load was undetectable 27 days after haplo-SCT, and MRI findings showed no apparent residual disease. This case highlights the importance of considering CNS relapse despite apparent virologic response in adult-onset EBV+ T-cell LPD. To our knowledge, this is among the few reported adult cases with clinically diagnosed CNS involvement successfully treated with allogeneic SCT.
Introduction
Epstein-Barr virus (EBV)-associated lymphoproliferative disorder (EBV-LPD) is a heterogeneous group of diseases characterized by the abnormal proliferation of EBV-infected lymphocytes, including B, T, or natural killer (NK) cells. These conditions range from relatively indolent or self-limiting presentations to fulminant disorders with extensive tissue infiltration and life-threatening complications, including infectious mononucleosis (IM)-like syndromes, post-transplant LPDs, chronic active EBV disease (CAEBV), and various EBV-positive lymphomas. CAEBV, a particularly rare and aggressive subtype, is typically caused by EBV-infected T or NK cells. It is characterized by persistent or recurrent inflammatory symptoms and elevated EBV-DNA levels in affected tissues. In Japan, the Ministry of Health, Labour and Welfare defines CAEBV based on strict clinical criteria, including IM-like symptoms persisting for more than three months, sustained EBV-DNA elevation in peripheral blood, and the presence of EBV-infected T or NK cells in either tissue or blood.
In contrast, the current international classifications, namely, the 5th edition of the WHO Classification of Haematolymphoid Tumors and the International Consensus Classification, also refer to prolonged IM-like symptoms but define CAEBV as part of a broader spectrum of EBV-positive T/NK-cell LPDs. The WHO framework emphasizes pathological and immunophenotypic features, and although it includes EBV-DNA elevation or EBV-encoded RNA (EBER)-positive cells in affected tissues as essential criteria, it does not mandate a specific EBV-DNA threshold or require detection in peripheral blood, as stipulated in the Japanese guidelines1, 2. Nevertheless, some EBV-LPDs show rapid progression and severe organ involvement, mimicking CAEBV both clinically and pathologically. In children, systemic CAEBV often involves the central nervous system (CNS) and has a poor prognosis3,4. In contrast, CNS involvement in adult-onset EBV-LPD is rare, and its clinical features and optimal management remain poorly understood. Here, we present the case of a 59-year-old woman with EBV-positive T-cell lymphoproliferation who developed CNS involvement during disease relapse. Although the case did not meet the formal criteria for CAEBV, the clinical course suggested a malignant EBV-driven process. The patient was successfully treated with haploidentical stem cell transplantation (haplo-SCT) and post-transplant cyclophosphamide (PTCy).
Case Presentation
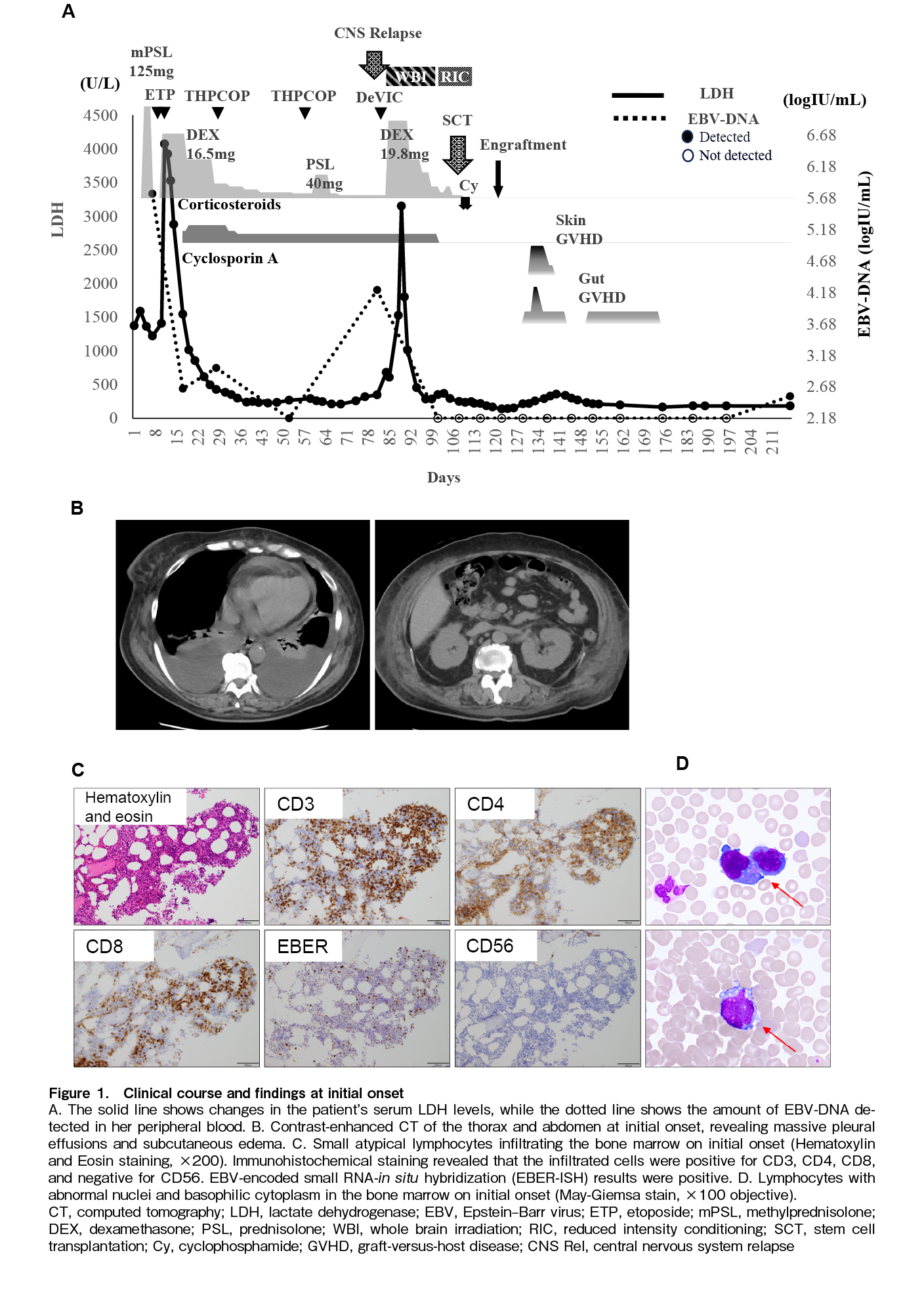
A 59-year-old woman was referred to our hospital with a chief complaint of fatigue, along with a 2-week history of persistent, high-grade fever; progressively worsening dyspnea; and systemic edema. She had no known immunodeficiencies, malignancies, or autoimmune diseases. There was no history of hypersensitivity to mosquito bites or photosensitivity. Baseline laboratory findings, including elevated levels of lactate dehydrogenase LDH (1,373 U/L) and soluble interleukin-2 receptor, are summarized in
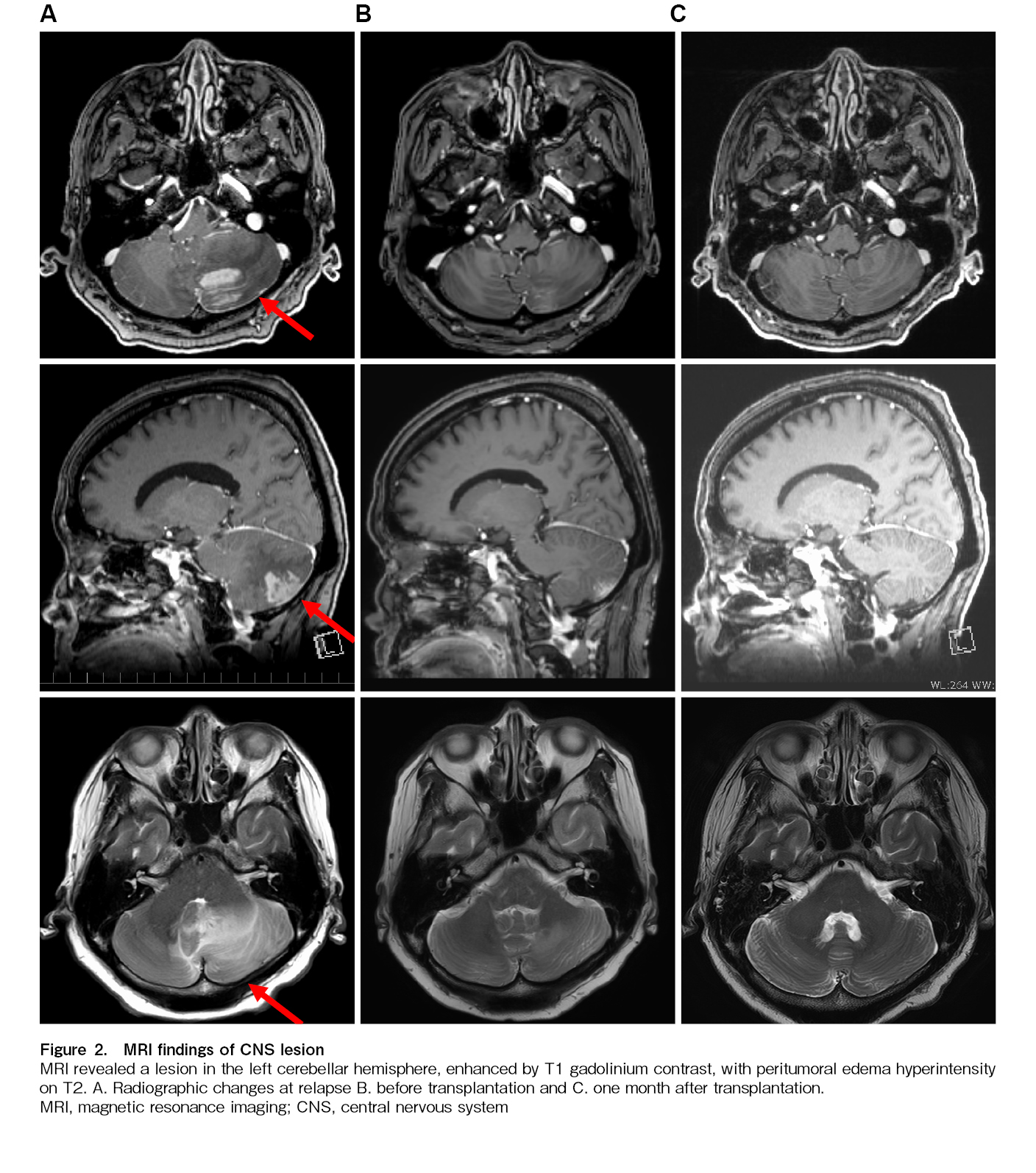
Twenty-two days after THP-COP second course initiation, at the time of hematologic recovery and in preparation for transplantation, the patient experienced systemic fatigue similar to that at initial presentation, accompanied by dizziness, gait unsteadiness, and horizontal nystagmus. Brain magnetic resonance imaging (MRI) subsequently demonstrated a gadolinium-enhancing lesion in the left cerebellar hemisphere (Figure 2A). EBV-DNA was detected (2.54 log IU/mL) and LDH level was elevated (139 U/L) on cerebrospinal fluid examination. Blood tests showed disease progression, with elevated EBV-DNA (4.22 log IU/mL) and LDH levels (682 U/L;
The reduced-intensity conditioning regimen was initiated on day 16 of DeVIC therapy, immediately after completion of whole-brain irradiation. We modified the conditioning regimen by adding high-dose cytarabine and administered haplo-SCT with PTCy (40 mg/kg for 2 days), following a reduced-intensity conditioning regimen (fludarabine, 30 mg/m2 for 6 days; busulfan, 3.2 mg/kg for 2 days; cytarabine, 2,000 mg/m2 for 2 days; and total body irradiation at 4 Gy) along with mycophenolate mofetil and tacrolimus. Peripheral stem cells collected from her younger brother contained 6.6×106/kg CD34-positive cells. Cyclosporine, which had been administered since the cooling therapy, was discontinued before the start of the conditioning regimen, and steroids were terminated 2 days before the SCT. No adverse CNS events occurred during conditioning. Oral mucositis and diarrhea appeared as regimen-related toxicities but were controlled via symptomatic treatment. Neutrophil engraftment was observed on day 16 post-transplant, and fluorescence in situ hybridization of the X/Y chromosome revealed complete donor chimerism. However, the patient continued to experience fever, erythematous papules, and diarrhea. Stage 1 gut graft-versus-host disease (GVHD) and stage 3 skin GVHD were diagnosed via lower gastrointestinal endoscopy and biopsy. Both were mildly relieved with topical steroids and non-specific treatments. Peripheral blood EBV-DNA remained undetectable 27 days after haplo-SCT, and MRI showed no residual disease (Figure 2C). Mycophenolate mofetil was administered until day 45. The patient was discharged on day 46 and achieved a complete metabolic response on day 103, as assessed using PET-CT. EBV DNA was detected in peripheral blood for approximately four months from day 107 but subsequently disappeared without intervention and was still not detected at one and a half years after transplantation. Tacrolimus tapering was initiated on day 75 and gradually accelerated thereafter, possibly allowing graft-versus-lymphoma effects to emerge and contributing to subsequent viral clearance. It was eventually discontinued on day 310.
Discussion
CNS involvement in EBV-LPD or CAEBV disease remains poorly understood, particularly in adults. Pediatric data suggest that CNS involvement is not uncommon and is associated with poor outcomes; however, adult cases are rare and often only identified postmortem7,8. To the best of our knowledge, only one adult case of CAEBV or EBV-LPD with CNS involvement has been reported, confirmed by pathological autopsy8. Further studies are required to predict CNS relapse in patients with EBV-LPD or CAEBV disease.
Although CAEBV disease is defined by persistent or recurrent symptoms, elevated EBV-DNA levels in T or NK cells, and histological evidence of EBV-driven proliferation, the patient did not meet the formal criteria due to the symptom duration. However, the rapid progression, systemic inflammation, and presence of EBV-infected T cells with atypical morphology strongly suggested a malignant EBV-driven lymphoproliferative process. Although the present case did not fulfill the formal diagnostic criteria for CAEBV, the clinical and pathological findings were consistent with an EBV-driven T-cell LPD within the CAEBV spectrum. Therefore, we adopted the term “T-cell LPD” in the title to emphasize the T-cell origin of EBV infection. This designation also acknowledges the distinction between CAEBV, which typically involves T or NK cells, and other EBV-LPDs such as post-transplant LPDs, which predominantly affect B cells. Oshima et al. showed that EBV-infected lymphocytes can evolve from a polyclonal to a monoclonal population, eventually leading to aggressive conditions such as lymphoma or hemophagocytic lymphohistiocytosis9. This concept suggests a dynamic spectrum of diseases, in which EBV-LPD, CAEBV, and EBV-positive T/NK cell lymphomas may represent different stages of a common pathological process.
The efficacy of allogeneic hematopoietic stem cell transplantation (allo-HSCT) in EBV-LPD with CNS involvement is uncertain. In this case, whole-brain irradiation and salvage chemotherapy were administered prior to transplantation, which may have contributed to disease control. Although evidence is limited, these findings suggest that CNS-targeted interventions should be considered in selected cases prior to allo-HSCT. The timing of HSCT is also critical. Although allo-HSCT is the only curative option for CAEBV, represented by EBV-LPD, its application is often delayed due to donor search and transplant logistics. The patient achieved apparent disease control after the second course of chemotherapy but relapsed suddenly before the transplant could proceed. This highlights the unpredictability of disease progression and the need for prompt transplantation once partial remission is achieved6,10–13. To overcome donor limitations and accelerate treatment, we chose haplo-SCT with PTCy. This approach has gained traction in recent years and offers a viable alternative to matched sibling or unrelated donor transplantation, particularly in aggressive lymphoid malignancies. In this case, haplo-SCT resulted in durable remission of both systemic and CNS disease, although its long-term efficacy in EBV-LPD requires further study14. Additionally, EBV-positive B-cell LPDs involving the CNS have been reported under immunosuppressive conditions15,16. Although pathogenetically distinct, these cases highlight the broader potential for EBV-driven CNS pathology across different immune contexts.
We report a rare case of adult-onset EBV+ T-cell LPD with CNS involvement that presented with features suggestive of CAEBV but did not meet its diagnostic criteria. This case highlights the importance of early intervention, including CNS-directed therapy and timely HSCT. Haplo-SCT with PTCy may be an effective treatment option in such cases, especially when rapid disease control is required. Future studies should aim to clarify diagnostic thresholds, identify risk factors for CNS relapse, and determine the optimal timing and modality of transplantation in EBV-associated lymphoproliferative disorders.
Acknowledgments
This study was partially supported by a grant from JSPS KAKENHI (Grant Number24K11563). The authors would like to thank the medical, nursing, laboratory, and clinical staff at the participating centers for their important contributions to this study and dedicated patient care.
Author Contributions
Conception and design: AW, HT; Provision of study materials or patients: All authors; Collection and assembly of data: AW, HT; Data analysis: AW, HT; Interpretation of data: All authors; Manuscript writing: HT, AW; Final approval of manuscript: All authors.
Conflicts of Interest
HT has received honoraria from AstraZeneca, Bristol Myers Squibb, Chugai Pharmaceutical, Eisai, Ono, Nihon Shinyaku, Novartis, SymBio Pharmaceuticals Limited, Takeda Pharmaceutical, Meiji Seika Pharma, Gilead Sciences, and AbbVie Inc. as well as patents and royalties from Mesoblast. KN has received honoraria from Meiji Seika, Janssen, Chugai, Bristol Myers Squibb, Kyowa Kirin, AbbVie, Daiichi Sankyo, and Ohara as well as research funding from Kyowa Kirin and Chugai. The other authors declare no conflicts of interest.
Acknowledgments
This study was partially supported by a grant from JSPS KAKENHI (Grant Number24K11563). The authors would like to thank the medical, nursing, laboratory, and clinical staff at the participating centers for their important contributions to this study and dedicated patient care.
Ethics Approval
This study was approved by the Institutional Review Board of Kumamoto University Hospital (approval no. 499) and was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from the patients involved.
Acknowledgments
This study was partially supported by a grant from JSPS KAKENHI (Grant Number24K11563). The authors would like to thank the medical, nursing, laboratory, and clinical staff at the participating centers for their important contributions to this study and dedicated patient care.
Data Availability
Inquiries for data should be directed to tatetsu@kumamoto-u.ac.jp, and the necessary data will be made available to achieve the aims of any approved proposal.
References
1.Yonese I, Sakashita C, Imadome KI, Kobayashi T, Yamamoto M, Sawada A, et al. Nationwide survey of systemic chronic active EBV infection in Japan in accordance with the new WHO classification. Blood Adv. 2020; 4: 2918-26.
2.Kimura H, Cohen JI. Chronic Active Epstein-Barr Virus Disease. Front Immunol. 2017; 8: 1867.
3.Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022; 36: 1720-48.
4.Campo E, Jaffe ES, Cook JR, Quintanilla-Martinez L, Swerdlow SH, Anderson KC, et al. The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee. Blood. 2022; 140: 1229-53.
5.Arai A. Advances in the Study of Chronic Active Epstein-Barr Virus Infection: Clinical Features Under the 2016 WHO Classification and Mechanisms of Development. Front Pediatr. 2019; 7: 14.
6.Sawada A, Inoue M, Kawa K. How we treat chronic active Epstein-Barr virus infection. Int J Hematol. 2017; 105: 406-18.
7.Ou W, Zhao Y, Wei A, Ma H, Zhang L, Lian H, et al. Chronic Active Epstein-Barr Virus Infection With Central Nervous System Involvement in Children: A Clinical Study of 22 Cases. Pediatr Infect Dis J. 2023; 42: 20-6.
8.Kobayashi Z, Tsuchiya K, Takahashi M, Yokota O, Sasaki A, Bhunchet E, et al. An autopsy case of chronic active Epstein-Barr virus infection (CAEBV): distribution of central nervous system (CNS) lesions. J Neurol Sci. 2008; 275: 170-7.
9.Ohshima K, Kimura H, Yoshino T, Kim CW, Ko YH, Lee SS, et al. Proposed categorization of pathological states of EBV-associated T/natural killer-cell lymphoproliferative disorder (LPD) in children and young adults: overlap with chronic active EBV infection and infantile fulminant EBV T-LPD. Pathol Int. 2008; 58: 209-17.
10.Kawa K, Sawada A, Sato M, Okamura T, Sakata N, Kondo O, et al. Excellent outcome of allogeneic hematopoietic SCT with reduced-intensity conditioning for the treatment of chronic active EBV infection. Bone Marrow Transplant. 2011; 46: 77-83.
11.Yamamoto M, Sato M, Onishi Y, Sasahara Y, Sano H, Masuko M, et al. Registry data analysis of hematopoietic stem cell transplantation on systemic chronic active Epstein-Barr virus infection patients in Japan. Am J Hematol. 2022; 97: 780-90.
12.Kimura H, Ito Y, Kawabe S, Gotoh K, Takahashi Y, Kojima S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012; 119: 673-86.
13.Arai A, Sakashita C, Hirose C, Imadome KI, Yamamoto M, Jinta M, et al. Hematopoietic stem cell transplantation for adults with EBV-positive T- or NK-cell lymphoproliferative disorders: efficacy and predictive markers. Bone Marrow Transplant. 2016; 51: 879-82.
14.Maegaki M, Kawamura K, Hara K, Hosoda R, Suzuki S, Hosoda Y, et al. Successful HLA-haploidentical stem cell transplantation with post-transplant cyclophosphamide in an older patient with chronic active Epstein-Barr virus infection. Int J Hematol. 2022; 116: 630-4.
15.O'Neill BP, Vernino S, Dogan A, Giannini C. EBV-associated lymphoproliferative disorder of CNS associated with the use of mycophenolate mofetil. Neuro Oncol. 2007; 9: 364-9.
16.Balaguer-Rosello A, Piñana JL, Bataller L, Montoro J, Romero S, Navarro I, et al. Central Nervous System Involvement in Epstein-Barr Virus-Related Post-Transplant Lymphoproliferative Disorders after Allogeneic Hematopoietic Stem Cell Transplantation. Transplant Cell Ther. 2021; 27: 261.e1-7.
Search
News