Volume 8 (2025) Issue 4 No.8 Pages 301-310
Abstract
Introduction
Multiple myeloma (MM) is a hematologic malignancy characterized by uncontrolled plasma cell proliferation in the bone marrow, leading to complications such as anemia, renal failure, and skeletal lesions1. It causes overpopulation of abnormal clonal plasma B cells in bone marrow, bringing down regulation of osteoblasts and activation of osteoclasts, which induce malignant bone lesions, kidney injury, anemia, hypercalcemia, and painful fractures2.
MM has attracted heightened interest among medical scholars and clinical practitioners due to its alarming global prevalence and the notable trends indicated in recent epidemiological studies. Considered as the second most common hematologic cancer across the world, MM accounted for 176,404 cases, representing 14% of all incidences in leukemia, lymphoma, and MM combined in 20203,4. The worldwide incidence of MM has been reported at approximately 160,000 cases, with mortality reaching around 106,0005. This rising trend is particularly pronounced in men and individuals aged 50 years or older, especially those residing in high-income countries, although incidences are also increasing in low-income nations6.
Autologous hematopoietic stem cell transplantation (AHSCT) remains a cornerstone in the management of eligible patients7. AHSCT is a medical procedure where a patient's own stem cells are collected, stored, and then re-infused into their body after a high-dose chemotherapy regimen to help repopulate their bone marrow and immune system. This is typically preceded by conditioning regimens designed to eradicate malignant plasma cells and facilitate engraftment of the transplanted stem cells.
Traditionally, standard-dose melphalan (SDM, melphalan [MEL]-200 mg/m2) has been regarded as the standard conditioning regimen used in AHSCT8. Its high toxicity, however, limits its use in certain patient populations like the elderly. Other potential adverse effects of SDM include prolonged bone marrow suppression, nausea, vomiting, diarrhea, alopecia, rash, pruritus, mouth ulceration, hypersensitivity reactions, mucositis, infections (bacteremia, pneumonia, Clostridium difficile, fungal infection, sepsis, septic shock), vascular disorders, and thromboembolic events (pulmonary embolism, ischemic cardiopathy, ischemic stroke)9.
In light of these concerns, reduced-dose melphalan (RDM, MEL-140 mg/m2) has emerged as a potential alternative11. Early studies showed no statistical difference between SDM and RDM in terms of overall survival (OS), progression-free survival, cumulative evidence of relapse, non-relapse mortality, hematopoietic recovery, and second primary malignancy rates. A retrospective cohort study also showed that AHSCT among patients in very good partial response (VGPR) or complete response (CR) significantly favored RDM over SDM in terms of overall survival12.
Nevertheless, the body of evidence comparing RDM to SDM remains inconclusive. Several studies have reported favorable outcomes with reduced dosage, but inconsistencies in patient populations, sample sizes, and treatment protocols make it difficult to draw definitive conclusions about its comparative efficacy and safety. To the best of the researchers' knowledge, there are also limited large-scale randomized controlled trials (RCTs) directly comparing the two regimens.
It is therefore necessary to consolidate available evidence. The need for a systematic review and meta-analysis of the available data is crucial to clarify whether RDM can serve as a viable alternative to SDM for patients with multiple myeloma. By synthesizing the findings from various studies, this study aims to provide a clearer understanding of the potential advantages and limitations of RDM, with the goal of informing clinical decision-making and improving patient outcomes.
Research questions
1. Is there a significant difference between RDM compared to SDM in terms of OS and progression-free survival (PFS) as conditioning regimens for autologous hematopoietic stem cell transplantation in patients with multiple myeloma?
2. What are the differences between reduced-dose and standard-dose melphalan regarding non-relapse mortality (NRM), transplant-related mortality (TRM), and engraftment time (ET) in patients undergoing AHSCT for MM?
Materials and Methods
This paper was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 Expanded Checklist to ensure adherence to standard practice of reporting systematic reviews and meta-analysis13.
Eligibility criteria
In choosing studies for this review, the researchers established the following criteria: (1) the studies must compare RDM with SDM as a single-agent conditioning regimen in AHSCT for MM, regardless of the disease status at the time of transplantation; (2) in case of tandem transplants for MM, only the first autologous HSCT will be included; (3) subjects of the study must be adult patients; (4) studies must have been published in peer-reviewed academic and/or medical journal; and (5) studies must have been published within the past decade (2015-2025). Studies not meeting these criteria were excluded, such as those dealing with younger patients; focusing on alternative conditioning regimens; those involving tandem, second, or subsequent transplants; and those published before 2015.
Search strategy and selection process
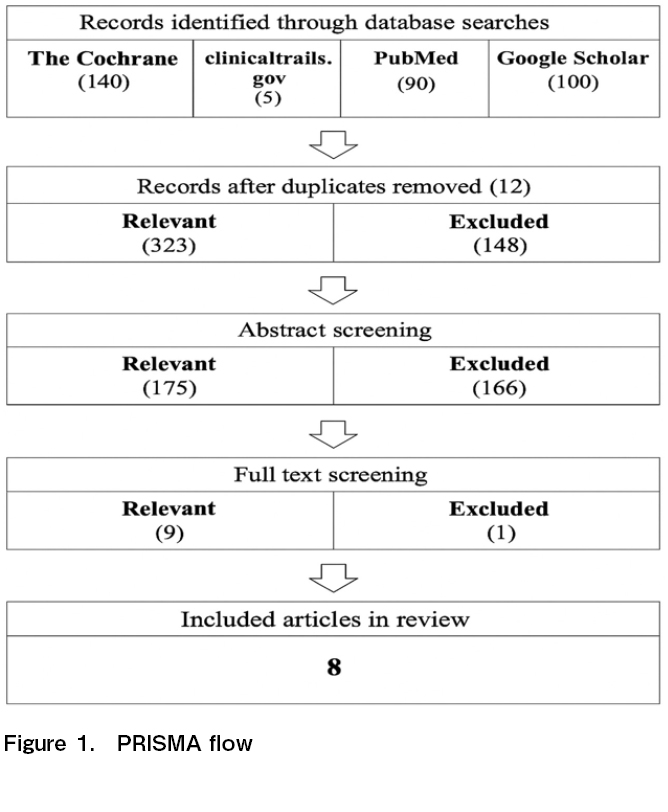
The systematic search was first conducted for this review on April 14, 2024 and updated on January 25, 2025 and August 11, 2025. The researchers focused on publications listed in electronic databases such as The Cochrane Library, ClinicalTrials.gov, PubMed Central, and Google Scholar. The entire PRISMA Flow is illustrated in Figure 1 in the succeeding chapter.
The researchers initially employed a broad search strategy to maximize the level of sensitivity in identifying potential related literatures about autologous hematopoietic stem cell transplantation for multiple myeloma with two dosage groups14. The researchers then narrowed down searches using MeSH terms and Boolean operators (OR & AND) to combine various components of the keywords to look for resources in the electronic databases mentioned above. The keywords included: multiple myeloma, myeloma, MM, melphalan 200 vs 140/100 conditioning, autologous HSCT, AND/OR autologous stem cell transplantation. The search was limited to studies in English published within the last 10 years.
The study selection process consisted of two stages: title/abstract screening and full-text screening. The researchers first looked into the title and read through the abstract to screen whether the variables involved in the resources were of any significance to the research at hand. A total of 335 studies were identified in the initial search but were significantly trimmed after the two-stage screening process, mainly due to duplicate copies, unavailability of full text, and not meeting the inclusion criteria.
The entire process of study selection is illustrated in Figure 1 covering the database used, the number of records identified, the number of records screened, the number of records excluded after screening titles/abstracts, the number of reports retrieved for detailed evaluation, the number of potentially eligible reports that were not retrievable, retrieved reports but did not meet inclusion criteria, and finally, the number of studies included in the review.
Outcome parameters
The primary outcome measures used to assess the efficacy of melphalan between two dosage groups (reduced vs standard-dose) were OS and PFS. Secondary outcome parameters included NRM, TRM, ET, and TRT.
Data collection process
The researchers extracted data by thematically categorizing the following: reference components (author, publication year, publisher, research design); participant characteristics (sample size, demographics); efficacy comparison in terms of OS and PFS; as well as secondary outcome parameters such as NRM, TRM, ET, and TRT.
Study risk of bias assessment
The risk of bias was assessed for each included study using the Cochrane Collaboration's tool for assessing risk of bias. The assessment criteria included seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other biases. Each study was meticulously looked into, especially on the methodology section of each paper, to serve as basis for being graded either as
Synthesis methods
As the primary outcome measure, OS and PFS were synthesized through meta-analysis owing to it being consistently reported across all studies included. However, secondary outcome parameters (NRM, TRM and ET) were synthesized through a systematic review due to limited and inconsistent reporting. Available data for each secondary parameter was summarized in a tabular format and a narrative synthesis was provided to contextualize the findings.
Statistical synthesis methods
The researchers used R with the metafor package, a well-established software widely used for statistical computing and conducting meta-analyses. Outcome parameters including OS and PFS, were synthesized using quantitative techniques. Effect sizes were computed using hazard ratios and pooled using weighted averages, with weights proportional to the inverse of the variance, assuming a fixed-effects model. Hazard ratios were calculated from the raw data or estimates reported by the authors and their 95% confidence intervals (CIs) were derived for OS and PFS using reported p-value for significance tests, population sizes, and the methodology outlined by Cohen's framework for effect size estimation. Variances were derived based on these effect sizes, and standard errors (SEs) were calculated.
Heterogeneity of studies
In this study, heterogeneity was assessed using multiple statistical methods, including the I-squared (I2) statistic, Tau-squared (τ2) estimate, Chi-square test, and a visual forest plot (see Figure 2 and 3) for each primary outcome parameter (i.e., OS and PFS). The I2 statistic measures the percentage of total variation across studies that is due to heterogeneity rather than chance, with I2 value between 0% and 25% reflecting low heterogeneity, while values above 50% suggests high heterogeneity. However, said I2 is not an absolute measure of heterogeneity13. Hence, other statistical measures were employed like τ2 statistic, which quantifies the variance in effect sizes across studies, as well as the Chi-squared test to formally assess the presence of heterogeneity.
Sensitivity analysis
For this study, sensitivity analysis was performed through Influence Diagnostics complemented with Cook's Distance Plot and DFFITS Plot. Influences Diagnostics assesses the influence of individual studies on the results of a statistical model18. These diagnostics are used to identify influential data points, which, if removed, could cause a significant change in the model's outcome. The results of the Influence Diagnostics were supported by Cook's Distance Plot as well as the DFFITS Plot. The results of the sensitivity analysis indicated that the overall conclusions of the meta-analysis remained consistent, providing confidence in the robustness of the findings.
Reporting bias assessment
Publication bias was investigated by drawing a funnel plot and conducting Egger's Test to assess reporting bias in this study. The funnel plot and statistical tests indicated no significant evidence of reporting bias, suggesting that the included studies provide a fairly representative sample of available research on the topic.
Ethical Considerations
Informed consent was not needed in the conduct of this study. This paper only involved a systematic review and meta-analysis of selected articles that did not contain any patient data, with no direct contact with the study population. An exception for review by the University of the Philippines Manila Research Ethics Board (UPMREB) was also applied and granted based on the criteria for exemption in the National Ethical Guidelines for Research Involving Human Participants 2022 (provision 47, page 48), that is,
Results
Included studies
The PRISMA flow diagram (Figure 1) illustrates the systematic search and selection process undertaken to identify relevant studies for this review. A total of 335 studies were initially identified in the broad search. Duplicate copies were accounted for prior to title/abstract screening where 12 studies were excluded. The researchers then conducted a title screening, during which 148 studies were excluded; followed by abstract screening which excluded 166 studies. The exclusion of these studies was mainly due to unavailability of full text and failure to meet the specified inclusion criteria. Nine (9) studies underwent a detailed full-text evaluation, where the researchers carefully assessed each study to ensure alignment with the established criteria. However, one study was excluded in this phase mainly because of sampling demographics in the study which covered young patients, unavailability of data required in assessing outcome parameters, and being outdated from the 10-year recency timeframe established in the inclusion criteria.
Study characteristics
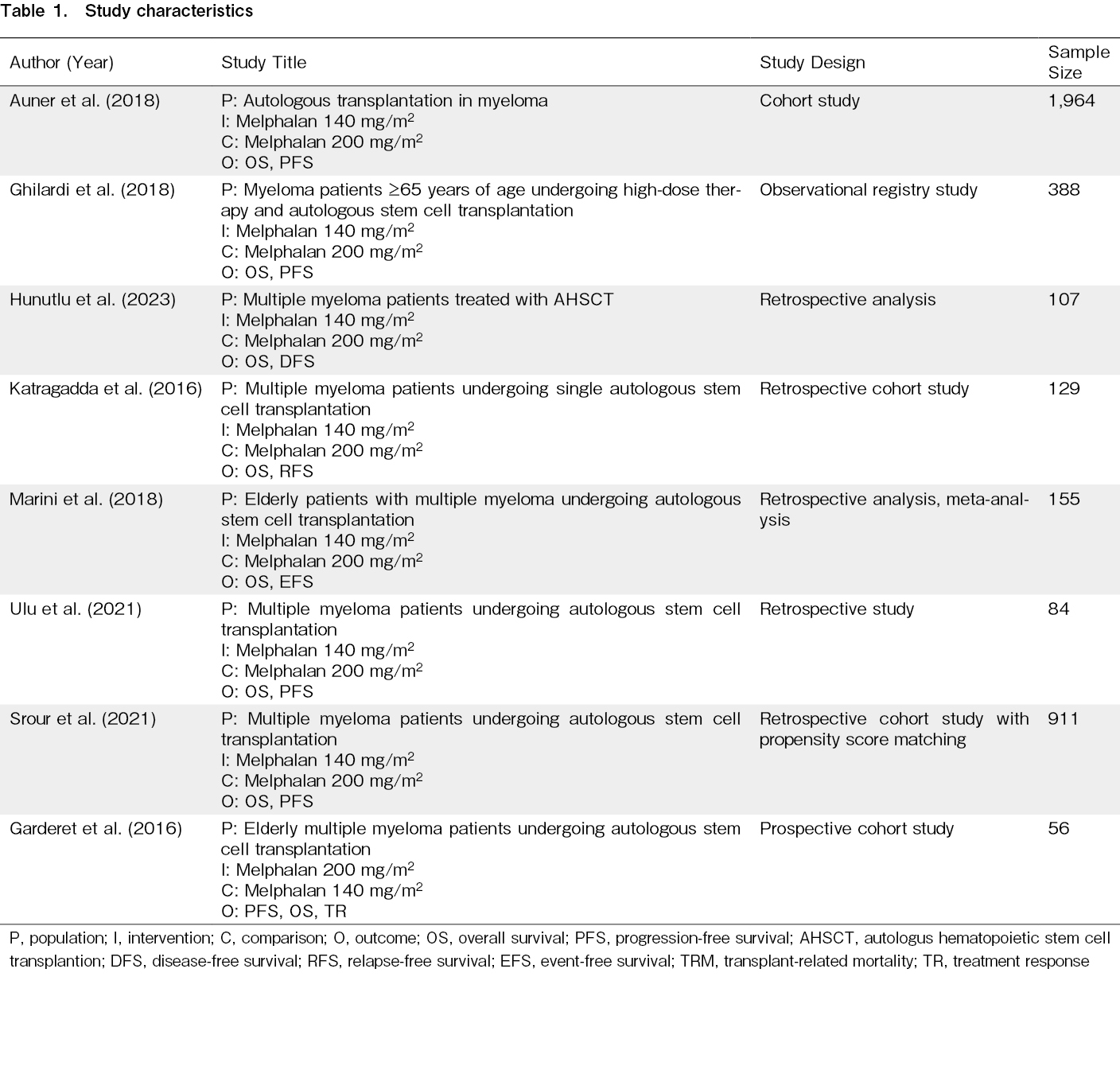
Table 1 summarizes the key attributes of the included research studies on the use of reduced versus standard-dose melphalan as a conditioning regimen in AHSCT for multiple myeloma. Overall, the studies span various design methodologies, including cohort (n=2), observational registry (n=1), and retrospective analyses (n=5). The combined sample size was 3,721, with the largest study involving 1,964 participants12.
Primary outcome parameters
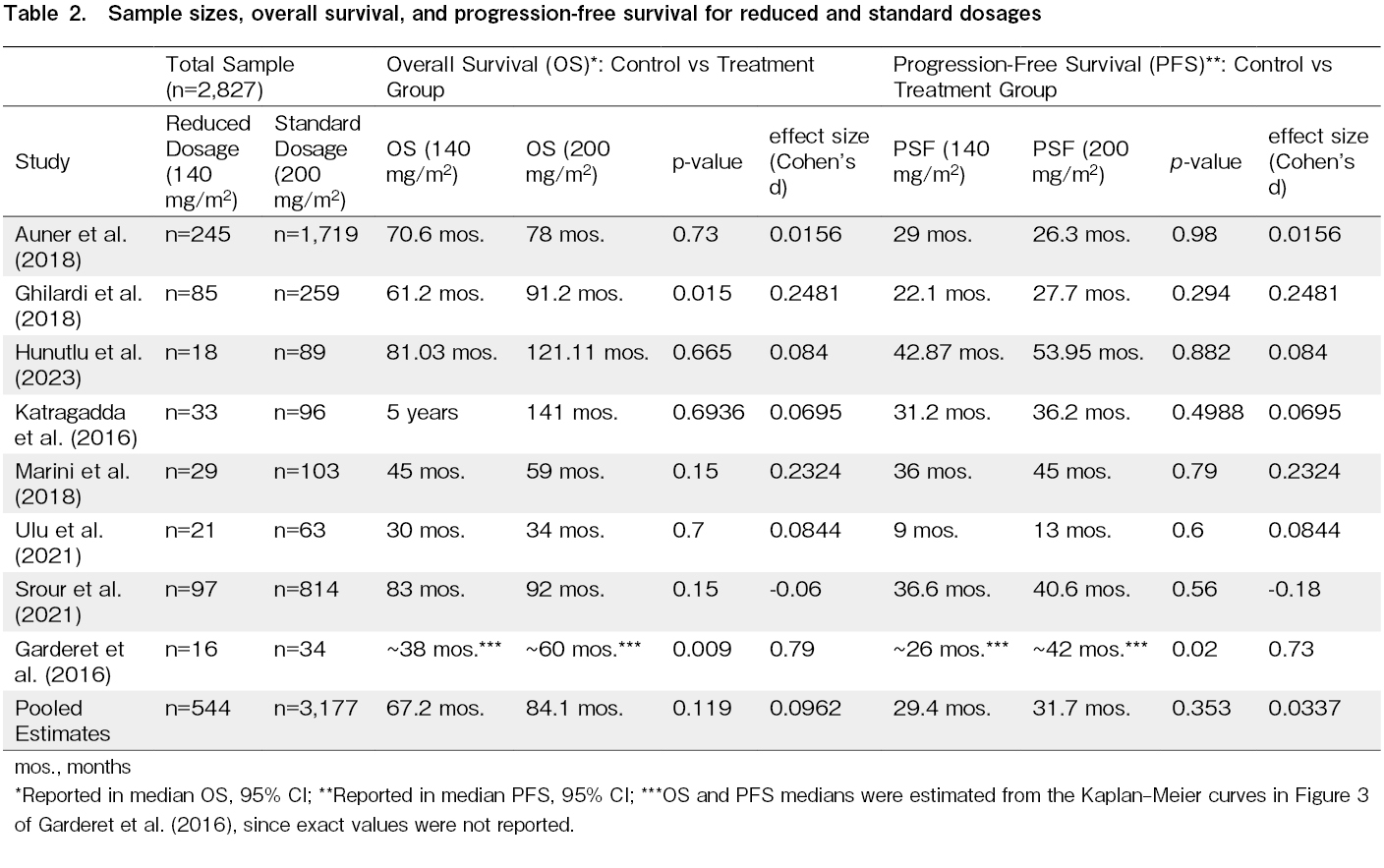
The data presented in Table 2 compares the efficacy of the control group (SDM) and treatment group (RDM) in terms of OS and PFS. The pooled median OS values for standard-dose and reduced-dose melphalan are 84.1 months and 67.2 months (95% CI), respectively, which implies a potential 16.9-month survival benefit for the standard-dose group. Similarly, the pooled median PFS values for standard-dose and reduced-dose melphalan are 31.7 months and 29.4 months (95% CI), respectively, suggesting a potential 2.3-month progression-free benefit for the standard-dose group. However, the observed differences are not statistically significant (p > 0.05) and may be due to chance rather than a genuine treatment effect. This is also captured in the pooled Cohen's d effect sizes of 0.0962 for OS and 0.0337 for PSF. These effect sizes indicate that the differences between the standard-dose and reduced-dose groups are modest at best.
Overall survival
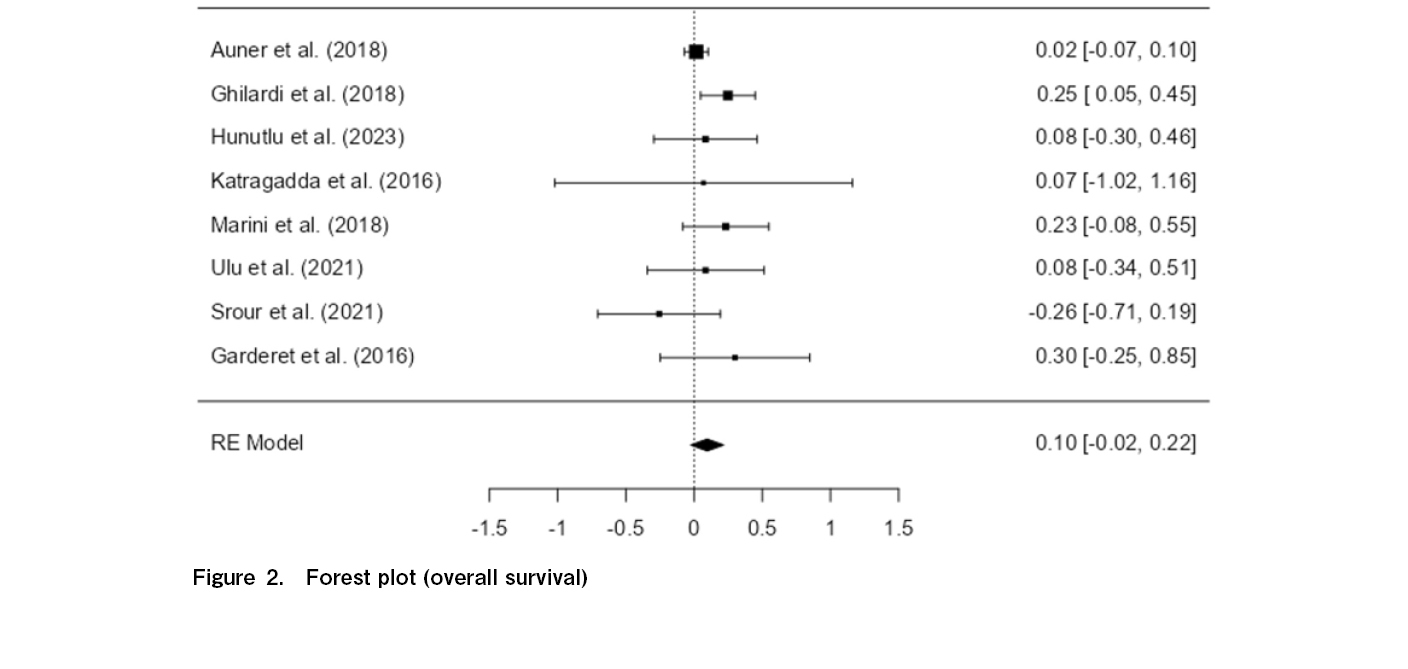
The current evidence―across 3,721 patient outcomes spanning eight studies included in this meta-analysis―does not support a statistically significant difference in OS between standard-dose and reduced-dose melphalan, with a p-value of 0.119 and an overall pooled effect size of 0.0962. The confidence interval (−0.025 to 0.217) crosses zero, suggesting that while there may be a small potential benefit to standard-dose melphalan, the true effect could be negligible. The Z-score of 1.56 (p > 0.05) also supports that the difference in OS between standard-dose and reduced-dose melphalan is not robust enough to support a firm conclusion regarding the superiority of one dosing regimen over the other.
As illustrated in Figure 2, moderate heterogeneity (I2=27.36%, p=0.316) was observed among the studies, indicating that the variability in results between the control and treatment groups is low-to-moderate and that the findings are reasonably consistent across all eight studies.
Progression-free survival
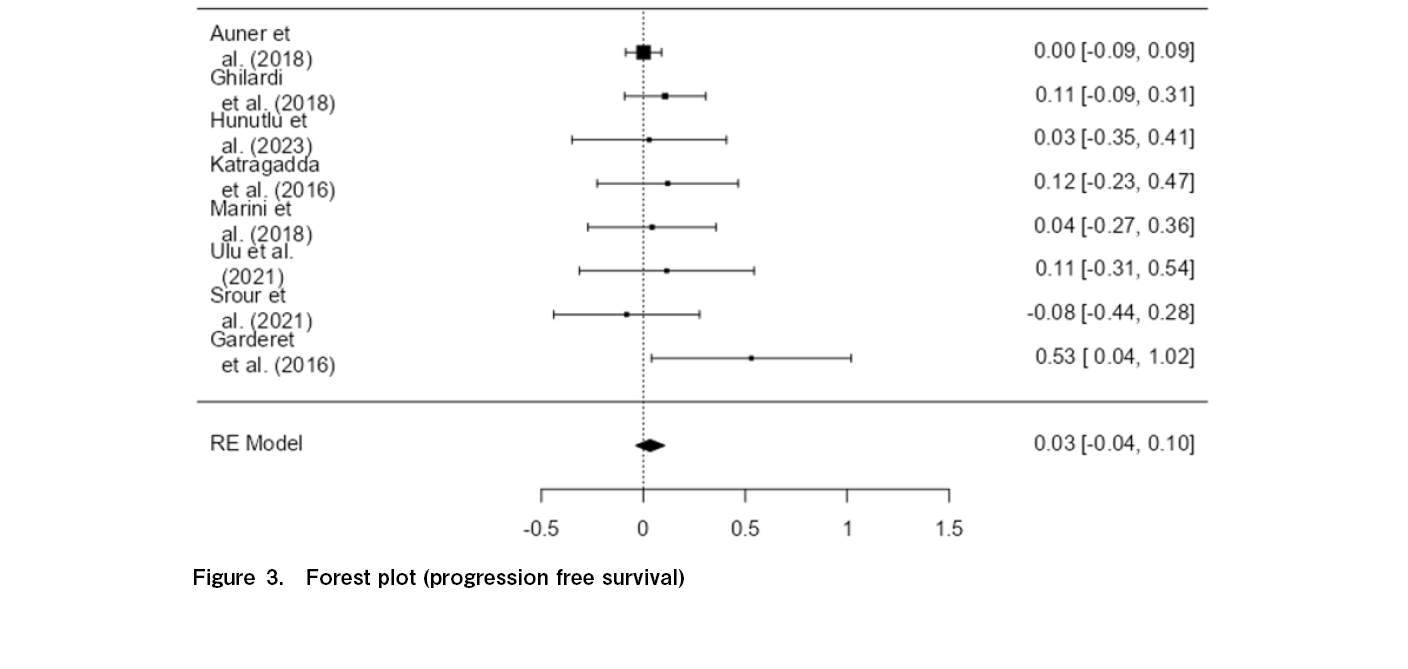
Figure 3 shows no statistically significant difference between the control group (SDM) and treatment group (RDM) in terms of PFS, as reflected by a p-value of 0.353 and a minimal effect size of 0.0337. While the calculated effect size suggests a very slight improvement in PFS for the standard-dose group, the standard error of 0.0363 combined with a confidence interval ranging from −0.037 to 0.105 indicates that this effect is not only modest but also includes the possibility of no difference. This finding suggests that the difference in PFS between the two dosing regimens is not clinically meaningful, supporting the notion that standard-dose melphalan does not offer a significant advantage over reduced-dose melphalan in prolonging the period before disease progression.
Furthermore, the figure above shows virtually no heterogeneity across the eight studies (I2=0.03%, Q=5.770, p=0.567), which confirms that the non-significant difference between the two dosages is consistent across all eight studies reviewed. There is also no significant publication bias affecting the study, as indicated by Egger's Regression (p=0.176) and Kendall's Tau (p=0.399).
Secondary outcome parameters
Non-relapse mortality
Among the eight studies included in the review, only two (2) studies addressed non-relapse mortality as an outcome parameter12, 27. In one study, a higher NRM rate was seen in RDM (1.3%) vs SDM (0.9%). However, such descriptive difference was not statistically significant (p=0.20)12. Conversely, another study reported an opposite trend, with MEL 140 showing lower NRM (1%) than MEL 200 (3%), also without statistical significance (p=0.64)27.
Transplant-related mortality
Four (4) studies have described TRM as an outcome parameter21,24,25,28. Among these studies, only one reported complete values where there were more TRM recorded within RDM group (2%) vis-à-vis SDM group (1.4%)21. This difference, however, may not necessarily be due to dosage but could only be due to chance as there were no statistically significant difference found between the two dosage groups in terms of TRM. This is also supported by two studies which reported 0 TRM in both dosage groups25,28. Meanwhile, one study only reported the overall TRM rate of 3% but did not differentiate between RDM and SDM groups24.
Engraftment time
Four (4) studies have reported outcomes in terms of engraftment time. Reports from these studies also made distinction between neutrophil engraftment and platelet engraftment expressed in number of days (median)22–25.
1. Neutrophil engraftment
In terms of neutrophil engraftment, the average engraftment time was at 11.7 days. No significant difference was reported between RDM and SDM groups (p > 0.05)22,25. However, one study described a significant difference (p < 0.05) was observed between the two groups in terms of neutrophil engraftment23. Meanwhile, another study reported a median of 12 days for both groups without differentiating between the two dosage groups28.
2. Platelet engraftment
For platelet engraftment, the analyses showed varying times to achieve thresholds of 20,000 and 50,000 platelets per microliter, with platelet recovery taking about 12 to 13 days in several studies. Across all four studies that reported quantitative details on platelet engraftment outcomes, no significant difference (p>0.05) was observed22–25.
Discussion
This paper's analysis―which covered outcomes from eight studies (published within the ten years as of today) with a total of 3,721 patients―showed no significant differences (p > 0.05) between the two dosage groups across primary indicators of OS and PFS. Likewise, systematic review on secondary outcome parameters, such as NRM, TRM, and ET, consistently showed no significant difference (p > 0.05). Of note, RDM was given to older patients, those who were frail, or had renal co-morbidities.
For the primary outcome parameters, analysis comparing SDM and RDM showed pooled estimate median OS at 84.1 months and OS 67.2 months, respectively. Average median PFS was 31.7 months vs 29.4 months for SDM vs RDM groups. Descriptive statistics showed a 16.9-month OS advantage and 2.3-month PFS benefit in favor of the SDM group. These, however, are statistically negligible. While there are no previously published meta-analyses for comparison of results, our findings suggest that the RDM remains as effective as the SDM due to several key factors.
Pharmacokinetics and efficacy at lower doses
Melphalan follows a dose-response relationship where higher doses increase cytotoxicity. However, once the threshold for maximum therapeutic effect is reached, further increasing the dose may not proportionally enhance efficacy but instead raise toxicity risks1. RDM may still achieve similar tumor-killing efficiency because the plasma concentration remains within the effective therapeutic window, ensuring sufficient exposure for myeloablation and disease eradication3.
Engraftment and hematopoietic Recovery
Engraftment success depends on factors beyond melphalan dose, such as stem cell dose, patient conditioning, and supportive care. Since engraftment times did not differ significantly between RDM and SDM, it suggests that a lower dose does not compromise bone marrow recovery2.
Reduced toxicity and improved tolerability
While SDM provides a slight numerical advantage in OS and PFS, the increased toxicity at higher doses may offset this benefit by leading to treatment-related complications, prolonged hospital stays, or increased mortality. Patients receiving RDM may experience fewer side effects, allowing for better adherence to post-transplant therapies and potentially improving long-term outcomes4.
Patient-specific factors and individualized dosing
RDM may be particularly beneficial for elderly patients or those with comorbidities, where high-dose chemotherapy poses significant risks. The lack of significant difference in survival outcomes suggests that individualized dosing based on patient tolerance can optimize efficacy while minimizing toxicity2.
Important insights on secondary outcome parameters were also obtained from the systematic review. NRM and TRM were both descriptively higher in RDM than in SDM but remained statistically not significant. However, the overall smaller sample size and inconsistent reporting of these parameters among the included studies preclude the researches from statistically analyzing possible trends and potential effects.
Review of neutrophil engraftment showed contradicting results reported by Hunutlu et al. (2023) and Ulu et al. (2021) compared to the study of Katragadda et al. (2016). This inconsistency may be due to the difference in population characteristics (RDM group in Katragadda et al. were older, had poorer cardiac and renal status, and inferior functional capacity). However, corresponding sample sizes of these studies are not enough to draw strong correlations.
Lastly, platelet engraftment was consistently reported between RDM and SDM among five studies (no significant difference at p > 0.05). While there was a substantial sample size among the five studies (n=525), this finding may reflect engraftment kinetics after high-dose melphalan + AHSCT (since both SDM and RDM are considered high-dose and myeloablative) rather than a true dose-dependent effect.
Overall, these findings reinforce the potential of RDM as a viable alternative to SDM, particularly in select patient populations where balancing efficacy and toxicity is crucial. The results of this meta-analysis significantly add to the growing body of evidence on optimum melphalan dose prior to AHSCT in multiple myeloma. On one hand, a study by Brioli et al. conluded that SDM does not increase toxicity and improves overall survival as opposed to RDM. This study even went on to argue based on their findings that SDM is still the standard of care for conditioning prior to AHSCT in multiple myeloma patients26. On the other hand, several studies with larger sample sizes have already challenged this proposition (i.e. Auner et al., n=1,964). This study showed no significant difference between the two dosage groups based on OS, PFS, cumulative incidence of relapse, NRM, hematopoietic recovery and second primary malignancy rates12.
However, these studies are primarily empirical in nature and a consolidation of studies across various clinical settings and locales for the past ten years have not yet been undertaken prior to this. To the best of the researchers' knowledge, this current meta-analysis, by far, has the widest sample size concerning this comparative clinical issue (n=3,721). Given current 10-year data, it is safe to conclude that there is no statical difference between patient outcomes of SDM and RDM. Hence, clinicians may consider patient-specific factors such as toxicity risk and tolerability when selecting the appropriate melphalan dosage, rather than relying on the assumption that higher doses necessarily lead to better patient outcomes.
Limitations and considerations
Despite the strengths of this meta-analysis such as
Firstly, there were limited studies for the past ten years comparing RDM and SDM as a single-agent conditioning regimen in AHSCT for multiple myeloma. After systematic search in four online databases, only eight studies fit within the inclusion criteria. These studies did not mention effects of consolidation and/or maintenance therapies after high-dose melphalan, which further limits this study.
Secondly, the lack of RCTs raises concerns on selection bias, confounding, and missing data. Since the studies reviewed were retrospective cohort studies, such limitations which can potentially affect causal inference is present. RCTs remain as the gold standard for assessing treatment efficacy as they minimize biases through randomization and controlled conditions.
Lastly, there was an inconsistent reporting of outcome parameters across studies. As discussed earlier, only primary outcomes (OS and PFS) were reported consistently across all studies, hence only these parameters were analyzed through quantitative synthesis/meta-analysis. Other outcome parameters like NRM, TRM, and ET could be found in some studies but were not reported in other studies, hence the synthesis was approached only through systematic review.
Conclusions and future directions
In summary, this systematic review and meta-analysis suggest that there is no statistically significant difference in OS or PFS between standard-dose and reduced-dose melphalan as a single-agent conditioning regiment prior to autologous stem cell transplantation in multiple myeloma. While the descriptive analysis points toward potential advantages linked to the higher dose, the statistical outcomes indicate that lower doses can be an alternative to minimize toxicity.
Future research is necessary to further explore factors influencing treatment decisions and outcomes. Large, randomized controlled trials with heterogeneous populations may provide clarity on specific subsets of patients who could benefit from either dosing strategy. Additionally, further analysis addressing long-term quality of life and the economic implications of treatment regimens could help guide clinicians in making informed treatment decisions. Ultimately, the choice between standard and reduced doses of melphalan should be individualized, incorporating patient preferences, health status, and potential treatment benefits versus risks.
Author Contribution
Both authors (RDY and DTV) were actively involved during the conceptualization, writing and editing of the protocol. Likewise, both authors performed article search, data analysis and development of the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Abduh MS. An overview of multiple myeloma: A monoclonal plasma cell malignancy's diagnosis, management, and treatment modalities. Saudi J Biol Sci. 2024; 31: 103920.
2.Antoine-Pepeljugoski C, Braunstein MJ. Management of Newly Diagnosed Elderly Multiple Myeloma Patients. Curr Oncol Rep. 2019; 21: 64.
3.Kundu S, Jha SB, Rivera AP, Flores Monar GV, Islam H, Puttagunta SM, et al. Multiple Myeloma and Renal Failure: Mechanisms, Diagnosis, and Management. Cureus. 2022; 14: e22585.
4.Huang J, Chan SC, Lok V, Zhang L, Lucero-Prisno DE 3rd, Xu W, et al. The epidemiological landscape of multiple myeloma: a global cancer registry estimate of disease burden, risk factors, and temporal trends. Lancet Haematol. 2022; 9: e670-7.
5.Ludwig H, Novis Durie S, Meckl A, Hinke A, Durie B. Multiple Myeloma Incidence and Mortality Around the Globe; Interrelations Between Health Access and Quality, Economic Resources, and Patient Empowerment. Oncologist. 2020; 25: e1406-13.
6.Hou Q, Li X, Ma H, Fu D, Liao A. A systematic epidemiological trends analysis study in global burden of multiple myeloma and 29 years forecast. Sci Rep. 2025; 15: 2204.
7.Bertolotto A, Martire S, Mirabile L, Capobianco M, De Gobbi M, Cilloni D. Autologous Hematopoietic Stem Cell Transplantation (AHSCT): Standard of Care for Relapsing-Remitting Multiple Sclerosis Patients. Neurol Ther. 2020; 9: 19-203.
8.Aypar E, İzzettin FV, Akı ŞZ, Sancar M, Yeğin Z, Türköz-Sucak G. Comparison of conditioning regimen toxicities among autologous stem cell transplantation eligible multiple myeloma patients: High-dose melphalan versus high-dose melphalan and bortezomib. J Oncol Pharm Pract. 2018; 24: 281-9.
9.Poczta A, Rogalska A, Marczak A. Treatment of Multiple Myeloma and the Role of Melphalan in the Era of Modern Therapies-Current Research and Clinical Approaches. J Clin Med. 2021; 10: 1841.
10.Savani BN, Mukherjee A, Savani GT, Ankit C. Utilization Trend and in-Hospital Complications of Autologous Hematopoietic Stem Cell Transplantation in Multiple Myeloma in the United States: 13 Years Perspective. Blood. 2014; 21: 3978.
11.El Fakih R, Fox P, Popat U, Nieto Y, Shah N, Parmar S, et al. Autologous Hematopoietic Stem Cell Transplantation in Dialysis-Dependent Myeloma Patients. Clin Lymphoma Myeloma Leuk. 2015; 15: 472-6.
12.Auner HW, Iacobelli S, Sbianchi G, Knol-Bout C, Blaise D, Russell NH, et al. Melphalan 140 mg/m2 or 200 mg/m2 for autologous transplantation in myeloma: results from the Collaboration to Collect Autologous Transplant Outcomes in Lymphoma and Myeloma (CALM) study. A report by the EBMT Chronic Malignancies Working Party. Haematologica. 2018; 103: 514-21.
13.Page MJ, Mckenzie MJ, Bossuyt PM, Boutron I, Hoffman TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372: n71.
14.Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods. 2017; 8: 5-18.
15.Driscoll JJ, Rixe O. Overall survival: still the gold standard: why overall survival remains the definitive end point in cancer clinical trials. Cancer J. 2009; 15: 401-5.
16.Walia A, Tuia J, Prasad V. Progression-free survival, disease-free survival and other composite end points in oncology: Improved reporting is needed. Nat Rev Clin Oncol. 2023; 20: 885-95.
17.Mostafaei H, Ghojazadeh M, Hajebrahimi S. Fixed-effect Versus Random-effects Models for Meta-analyses: Fixed-effect Models. Eur Urol Focus. 2023; 9: 691-2.
18.Iyengar S, Greenhouse JB. Sensitivity Analysis and Diagnostics. In: Cooper H, Hedges LV, Valentine JC, editors. The HANDBOOK OF RESEARCH SYNTHESIS AND META-ANALYSIS, 2nd ed. Russell Sage Foundation, 2009; 417-33.
19.Pustejovsky JE, Rodgers MA. Testing for funnel plot asymmetry of standardized mean differences. Res Synth Methods. 2019; 10: 57-71.
20.Page MJ, Sterne JA, Higgins JP, Egger M. Investigating and dealing with publication bias and other reporting biases in meta-analyses of health research: A review. Res Synth Methods. 2021; 12: 248-59.
21.Ghilardi G, Pabst T, Jeker B. Melphalan dose in myeloma patients ≥65 years of age undergoing high-dose therapy and autologous stem cell transplantation: A multicentric observational registry study. Bone Marrow Transplant. 2019; 54: 1029-37.
22.Hunutlu FÇ, Özkalemkaş F, Gürsoy V, Özkocaman V. Mel-200 or Mel-140, Which One is More Advantageous? Retrospectively Analysis of The Multiple Myeloma Patients Treated with Autologous Hematopoietic Stem Cell Transplantation. Turkish Journal of Internal Medicine. 2023; 5: 92-8.
23.Katragadda L, McCullough LM, Dai Y. Effect of melphalan 140 mg/m2 vs 200 mg/m2 on toxicities and outcomes in multiple myeloma patients undergoing single autologous stem cell transplantation-a single center experience. Clin Transplant. 2016; 30: 894-900.
24.Marini C, Maia T, Bergantim R, Pires J, Aguiar E, Guimarães JE. Real-life data on safety and efficacy of autologous stem cell transplantation in elderly patients with multiple myeloma. Ann Hematol. 2019; 98: 369-79.
25.Ulu BU, Bakırtaş M, Yiğenoğlu TN, Başcı S, Yıldız J, Şahi D, et al. Efficacy of reduced dose melphalan conditioning for multiple myeloma patients undergoing autologous stem cell transplantation: in the era of combined induction with novel agents. J Health Sci Med. 2021; 4: 203-8.
26.Brioli A, Vom Hofe F, Rucci P, Ernst T, Yomade O, Hilgendorf I, et al. Melphalan 200 mg/m2 does not increase toxicity and improves survival in comparison to reduced doses of melphalan in multiple myeloma patients. Bone Marrow Transplant. 2021; 56: 1209-12.
27.Srour SA, Milton DR, Bashir Q, Nieto Y, Saini N, Daher M, et al. Melphalan dose intensity for autologous stem cell transplantation in multiple myeloma. Haematologica. 2021; 106: 3211-4.
28.Garderet L, Beohou E, Caillot D, Stoppa AM, Touzeau C, Chretien ML, et al. Upfront autologous stem cell transplantation for newly diagnosed elderly multiple myeloma patients: a prospective multicenter study. Haematologica. 2016; 101: 1390-7.
Search
News