Volume 8 (2025) Issue 4 No.6 Pages 286-289
Abstract
Primary graft failure is an uncommon but serious complication that occurs when the hematopoietic engraftment fails to occur within the expected timeframe. Here, we report the case of a 63-year-old female with multiple myeloma who developed primary graft failure after Autologous stem cell transplant (ASCT). Despite initial concerns regarding his poor prognosis, the patient was successfully treated with cyclosporine as a novel rescue therapy. This condition necessitates prolonged hospitalization, frequent transfusions, and poses a high risk of life-threatening infections due to persistent cytopenias. The incidence of primary graft failure in myeloma patients undergoing ASCT has been reported to be less than 1% in recent studies. Despite its low occurrence, the clinical consequences of this complication are profound, contributing significantly to morbidity and mortality in this patient population. Cyclosporine has emerged as a promising therapeutic agent for the management of primary graft failure. The mechanism of action is believed to involve immunosuppression. This case underscores the potential utility of cyclosporine as a therapeutic option in patients with primary graft failure following ASCT, offering an alternative to the more invasive and risky strategy of allogeneic stem cell transplant.
Introduction
Multiple myeloma is a clonal plasma cell proliferative disorder characterized by the excessive production of monoclonal immunoglobulins, which can result in significant end-organ damage, including renal failure, bone lesions, and hematologic complications such as anemia1. The treatment landscape for multiple myeloma has evolved significantly, with autologous stem cell transplantation (ASCT) serving as the cornerstone for eligible patients with newly diagnosed disease. High-dose melphalan conditioning followed by stem cell rescue improves progression-free survival in these patients2. Despite the advancements in treatment, challenges remain in managing the rare but potentially devastating complications of ASCT.
Primary graft failure is an uncommon but serious complication that occurs when the hematopoietic engraftment fails to occur within the expected timeframe. It is defined by a peripheral blood absolute neutrophil count (ANC) of less than 500/μL and a platelet count of less than 20,000/μL by day 21 post-transplant3. Primary graft failure can result in prolonged pancytopenia, increased risk of infections, and ultimately, poor prognosis if left unaddressed. There are no universally accepted treatment strategies for this condition, and management remains challenging. Various approaches have been explored, including autologous stem cell boost (Cook et al., 2022), second autologous transplant, and allogeneic transplant (Pottinger et al., 2002). However, these interventions do not guarantee success and often come with their own risks and complications. Empirical cyclosporine therapy has been shown to promote neutrophil engraftment within a median of 5-7 days3,4, while eltrombopag is widely used for treating poor graft function post allogenic stem cell transplant5.
Here, we report the case of a 63-year-old female with multiple myeloma who developed primary graft failure after ASCT. Despite initial concerns regarding her poor prognosis, the patient was successfully treated with cyclosporine and eltrombopag as a novel rescue therapy. This case illustrates the potential of cyclosporine and eltrombopag combination as an effective strategy for reversing primary graft failure and highlights the need for further investigation into alternative therapies in such cases.
Case Report
A 63-year-old female was diagnosed with IgG kappa multiple myeloma in February 2024. At baseline, the patient had a Revised International Staging System (R-ISS) score of 2, with no high-risk cytogenetic abnormalities detected on fluorescence in situ hybridization (FISH). She received four cycles of induction therapy with the Velcade-Revlimid-Dexa (VRd) regimen (bortezomib 1.3 mg/m2 subcutaneously weekly, lenalidomide 20 mg once daily for 21 days of a 28-day cycle, and dexamethasone 40 mg weekly). After completing the induction, she achieved a very good partial response (VGPR) as per the International Myeloma Working Group (IMWG) criteria and proceeded to ASCT.
Pre-transplant evaluation was unremarkable, and the patient did not develop any infectious complications during induction therapy or prior to transplantation. Stem cell mobilisation was achieved with standard G-CSF plus Plerixafor protocol (G-CSF 10 mcg/kg for 5 days and Plerixafor 0.24 mg/kg 11 hours prior to apheresis) and CD34 dose of 11 million/kg was collected. Stem cell product was stored at 4 degree celsius in the blood bank for a period of 24 hours. Conditioning with melphalan (200 mg/m2) was administered after stem cell collection on the same day, followed by stem cell infusion 24 hours later at a CD34 dose of 11 million/kg. Growth factor support was initiated on day +2. Patient was also initiated on valacyclovir and fluconazole prophylaxis as per standard protocols from day +2. Antiemetics like Ondansetron and Metoclopramide were administred, when required.
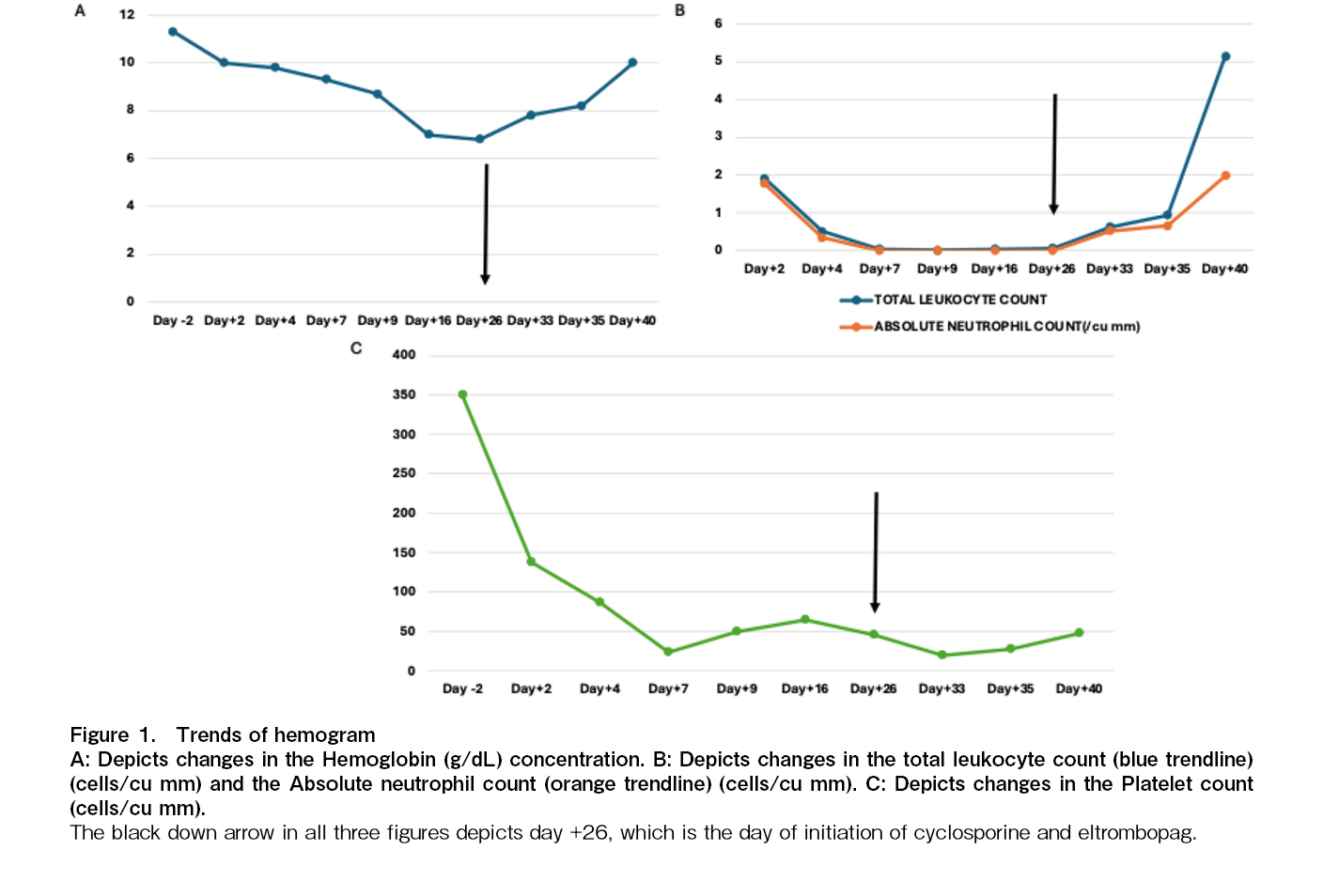
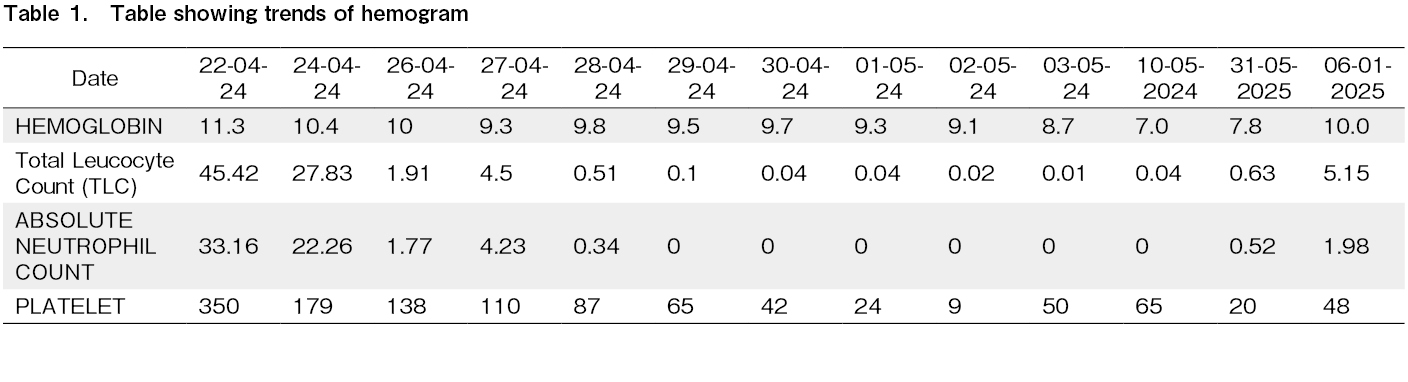
The patient developed Common Terminology Criteria for Adverse Events (CTCAE) grade 3 oral and abdominal mucositis starting on day +6, which was managed conservatively and resolved by day +13. Due to persistent cytopenia, a bone marrow biopsy was performed on day +24, revealing hypocellular marrow with less than 5% cellularity. Infective work up was done to rule out transient marrow aplasia. Cytomegalovirus (CMV), Epstein-Barr virus (EBV), Adenovirus, Parvovirus PCR was negative. Blood and Urine cultures were sterile and a CT scan of the chest did not reveal any active infection. In response, treatment with eltrombopag (150 mg once daily) and cyclosporine (3 mg/kg/day in two divided doses) was initiated on day
On day +30, the patient developed probable fungal pneumonia, which was managed with appropriate antifungal therapy. Neutrophil engraftment was achieved by day +35, and platelet engraftment was noted by day
The patient received one cycle of VRd consolidation starting on day +75 but developed recurrent cytopenias during lenalidomide therapy. A bone marrow biopsy on day +100 demonstrated minimal residual disease (MRD) negativity by flow cytometry (sensitivity of 10-4), and a PET-CT scan showed a complete metabolic response. Bortezomib maintenance therapy (2 mg subcutaneously every 15 days) was initiated on day +110.
As of the most recent follow-up, 8 months post-transplant, the patient remains in complete remission (CR).
Discussion
Primary graft failure is an exceedingly rare but often fatal complication in multiple myeloma patients undergoing ASCT. This condition necessitates prolonged hospitalization, frequent transfusions, and poses a high risk of life-threatening infections due to persistent cytopenias. The incidence of primary graft failure in myeloma patients undergoing ASCT has been reported to be less than 1% in recent studies3,6. Despite its low occurrence, the clinical consequences of this complication are profound, contributing significantly to morbidity and mortality in this patient population.
The pathogenic mechanisms and risk factors for graft failure in ASCT remain poorly understood. Several factors have been implicated in the delayed engraftment of hematopoietic stem cells, including stem cell dose and viability, the duration between diagnosis and transplant, the conditioning regimen, the effect of prior treatments on the bone marrow microenvironment, and peri-transplant infectious complications7. Lower CD34+ cell count (less than 3 million/kg), transfusion dependence before transplant, and low pre-transplant platelet count (less than 150,000/mm3) have been found to be statistically significant predictors of delayed neutrophil and platelet engraftment8. Additionally, the suppression of hematopoiesis by auto-reactive T lymphocytes is thought to contribute to primary graft failure, although the exact mechanism remains unclear3.
Empirical cyclosporine therapy has shown encouraging results in the treatment of primary graft failure post ASCT4. The mechanism of action is believed to involve suppression of autoreactive T lymphocytes, which reduces immune-mediated rejection of the graft and promotes hematopoietic recovery9. Eltromopag, a thrombopoeitin receptor agonist, has been reported to improve platelet counts and overall hematopoietic recovery in patients with poor graft function (PGF) post stem cell transplant5. In our case, the patient underwent ASCT following four cycles of chemotherapy with the VRd regimen. Pre-transplant blood counts were normal, and CD34 dose of 11 million/kg was considered adequate. Notably, no infectious complications arose in the first three weeks following the transplant. However, despite these favorable conditions, the patient experienced primary graft failure, and no apparent cause was identified.
Given the absence of cryopreserved stem cells for a potential stem cell boost, a trial of cyclosporine and eltrombopag was initiated prior to considering an allogeneic transplant. Neutrophil engraftment occurred on day +35, and platelet engraftment was achieved by day +40, after starting cyclosporine and eltrombopag. Remarkably, cyclosporine and eltrombopag could be tapered off after one month, suggesting that this approach facilitated hematopoietic recovery. However, since the neutrophil engraftment occurred by D+35, possibility of spontaneous recovery cannot be excluded in our patient. Similar positive outcomes were observed in a small study by Cubillas et al., where four out of five patients with primary graft failure after autologous transplant successfully engrafted following treatment with cyclosporine with median time of 5 days to achieve absolute neutrophil count (ANC) > 500/uL3.
This case underscores the potential utility of cyclosporine and eltrombopag as a therapeutic option in patients with primary graft failure following ASCT, offering an alternative to the more invasive and risky strategy of allogeneic stem cell transplant. Allogeneic transplant in this patient cohort has been associated with poor outcomes, including a high risk of graft-versus-host disease and relapse10. Therefore, cyclosporine with eltrombopag therapy should be considered as a first-line treatment in such cases, especially in the absence of other effective options.
Author Contributions
NMK: primary physician, RB and VD: team leads and supervision for treatment, administration and revision of manuscript, CY and AS: adult hematologists involved in patient care and data curation, SN: wrote the report, NRP infectious disease physician to manage infectious complications. MNK and SN have contributed equally.
Conflicts of Interest
The author declares no conflict of interest. Disclosure form provided by the author are available on the website.
References
1.Cowan A, Green D, Kwok M, Lee SS, Coffey D, Holmberg L, et al. Diagnosis and Management of Multiple Myeloma: A Review. JAMA. 2022; 327: 464-77.
2.Al Hamed R, Bazarbachi AH, Malard F, Harousseau J-L, Mohty M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019; 9: 44.
3.Hama Sphere, Cubillas D, Quesada Sanchez M, Martinez Sanchez P, Robles MC, Pina MS, et al. P1328 Succesful treatment of graft failure in autologous stem cell transplantation with cyclosporine. 2022. https://onlinelibrary.wiley.com/doi/10.1097/01.HS9.0000848176.34514.00 [Accessed: 13 March 2025]
4.Kamble RT, Sethi S, Selby GB. Re: failure to engraft after autologous stem cell transplantation: possible therapeutic role of cyclosporine. Biol Blood Marrow Transplant. 2005; 11: 74.
5.Halahleh K, Al-Ya'Goub M, Ma'koseh M, Al-Far R, Da'na W, Pharm RA, et al. Eltrombopag Enhances Recovery from Cytopenias Due to Poor Graft Function after Hematopoietic Cell Transplantation. Blood Cell Ther. 2025; 8: 160-6.
6.Cook J, Gonsalves WI, Gertz MA, Visram A, Warsame R, Lacy MQ, et al. Success of the autologous stem cell boost after autologous graft failure in multiple myeloma and AL amyloidosis. Bone Marrow Transplant. 2022; 57: 1007-9.
7.Ergene U, Cağirgan S, Pehlivan M, Yilmaz M, Tombuloğlu M. Factors influencing engraftment in autologous peripheral hematopoetic stem cell transplantation (PBSCT). Transfus Apher Sci. 2007; 36: 23-9.
8.Lutfi F, Skelton Iv WP, Wang Y, Rosenau E, Farhadfar N, Murthy H, et al. Clinical predictors of delayed engraftment in autologous hematopoietic cell transplant recipients. Hematol Oncol Stem Cell Ther. 2020; 13: 23-31.
9.Cardenas ME, Zhu D, Heitman J. Molecular mechanisms of immunosuppression by cyclosporine, FK506, and rapamycin. Curr Opin Nephrol Hypertens. 1995; 4: 472-7.
10.Yin X, Tang L, Fan F, Jiang Q, Sun C, Hu Y. Allogeneic stem-cell transplantation for multiple myeloma: a systematic review and meta-analysis from 2007 to 2017. Cancer Cell Int. 2018; 18: 62.
Search
News