Volume 8 (2025) Issue 4 No.1 Pages 252-261
Abstract
Despite advances in lymphoma treatment, autologous hematopoietic stem cell transplantation (auto-HCT) remains essential in regions with limited access to novel therapies. Improved survival post-auto-HCT has led to an increased incidence of therapy-related myeloid neoplasms (t-MN).
We conducted a retrospective analysis of adult patients (≥18 years) who underwent auto-HCT for lymphoma at King Hussein Cancer Center in Jordan between 2003 and 2020 with the goal of evaluating characteristics and outcomes of patients developing t-MN post-auto-HCT.
We identified 407 patients with a median follow-up of 5.8 years. The median age at auto-HCT was 34.6 years, 54.9% were males, 65.3% had Hodgkin lymphoma, and 41.1% were refractory to first-line treatment. dexamethasone, ara-C, cisplatin (DHAP) was the most common salvage regimen (47.8%). At the time of auto-HCT, 39.7% were in complete remission. BCNU, etoposide, ara-C, melphalan (BEAM) was the most common conditioning regimen 91.9%. The 5-year overall survival (OS) was 64.4%.
Thirteen patients (3.2%) developed t-MN (5 acute myeloid leukemia, 8 myelodysplastic syndrome) with a median onset of 3.25 years post-auto-HCT. The median OS post t-MN diagnosis was 6 months. Patients with t-MN were older (p < 0.001), more likely to have non-Hodgkin lymphoma (p=0.023) and had more comorbidities (p=0.02). The most common cytogenetic abnormality was del (7) (46%), and TP53 was the most common molecular abnormality (15%). Age at transplant was the only significant predictor of t-MN (Hazard Ratio=1.162, p < 0.001) on multivariate analysis. t-MM accounted for 18.8% of non-relapse mortality (NRM).
t-MN significantly contributes to NRM post-auto-HCT. Age at transplant is the primary risk factor, highlighting the need for vigilant monitoring and risk mitigation strategies.
Introduction
High-dose chemotherapy and autologous hematopoietic stem cell transplantation (auto-HCT) is a well-established treatment option for patients with relapsed Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL), especially in regions with limited access to novel treatments. With improved survival outcomes following auto-HCT, there has been a rise in therapy-related myeloid neoplasms (t-MN)1,2, a leading cause of non-relapse mortality (NRM)3–6. T-MN, which manifest as therapy-related myelodysplastic syndrome (t-MDS) or therapy-related acute myeloid leukemia (t-AML), represent a genetically heterogeneous and high-risk group of secondary myeloid disorders arising as late complications of cytotoxic therapies for primary malignancies7–9.
These disorders are characterized by complex genomic alterations, including TP53 mutations and cytogenetic abnormalities such as del(5q)/5- and del(7q)/7-, which contribute to their poor prognosis10,11. Several factors unique to auto-HCT for lymphoma patients influence the risk of developing t-MN. These include hematopoietic stem cell (HSC) mobilization stress, graft size, pre-existing clonal hematopoiesis, and the cumulative impact of chemotherapy prior to high-intensity conditioning regimens12–14. Reported incidences of t-MN range widely from 1.1% to 24.3%, reflecting differences in patient populations and treatment protocols13,15–19. This study evaluates the incidence and characteristics of t-MN following auto-HCT for lymphoma at King Hussein Cancer Center (KHCC), Jordan's leading cancer treatment facility, over two decades. By exploring these outcomes, this study provides insights into the long-term risks of auto-HCT and the impact of evolving treatment practices.
Materials and Methods
Study design and population
We conducted a systematic retrospective analysis of all adult patients (aged ≥ 18 years) who underwent auto-HCT for lymphoma at KHCC between January 2003 and December 2020. Data was initially obtained from KHCC HCT database and supplemented by additional data from electronic medical records.
Data collected included demographic information, patient age at lymphoma diagnosis, comorbidities, age at transplant, initial disease characteristics, pre-HCT treatments, and HCT characteristics and variables. For patients with t-MN, additional information included the date of t-MN diagnosis, clinical presentation, subtype (t-MDS or t-AML) and relevant cytogenetic and molecular data. Ethical approval for the study was obtained by KHCC's institutional review board (IRB approval 23 KHCC 133). The IRB at KHCC waived consent for conducting this study.
Definitions
We defined t-MN according to the 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia7. Molecular analysis was performed using a standard 54-myeloid gene panel using next-generation sequencing (NGS) with sensitivity of 5% variant allele fraction and a minimum depth of coverage of 500X. Cytogenetic evaluation was performed using conventional karyotyping and fluorescence in situ hybridization (FISH). Comorbidities were defined as presence of at least one of the following: hypertension, diabetes, coronary artery disease, cerebrovascular disease and chronic kidney disease. Overall survival (OS) for the entire cohort was defined as the time from HCT until death or last observation alive. Progression-free survival (PFS) was defined as the time from HCT until disease progression or death. NRM was defined as death without relapse of primary disease. OS for patients with t-MN was defined as the time from diagnosis of t-MN until death or last observation alive.
Statistical analysis
Continuous variables were summarized using the sample median and range and analyzed using the Mann-Whitney U test. Categorical variables were summarized with number and percentages were compared between groups using the chi-square test. Survival and time-to-event outcomes, including the development of t-MN, were estimated using the Kaplan-Meier method, with differences compared using the log-rank test. Multivariable analysis to identify independent risk factors for t-MN was conducted using Cox proportional hazards regression. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 28.0 (IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp). A p-value of ≤ 0.05 was considered statistically significant for all analyses.
Results
Patient and HCT characteristics
A total of 407 patients underwent auto-HCT for lymphoma at our center. The median follow-up for the entire cohort was 5.8 years (range 5-6.1). the median age at diagnosis of lymphoma was 31.8 years (range: 10.5-68.9) and the median age at the time of auto-HCT was 34.6 years (range 19-71), with 223 (54.9%) of the patients being male. Of the 407 patients 265 (65.3%) had HL, while 142 (34.9%) patients had NHL. The indication for auto-HCT was refractory disease in 167 (41.1%) and relapsed disease in 240 (58.9%). The most common salvage regimen used was a combination of dexamethasone, cisplatin, and cytarabine (DHAP) (n=194, 47.8%), followed by a combination of cisplatin, dexamethasone, and gemcitabine (GDP) (n=84, 20.6%), with 173 patients (42.6%) receiving more than one salvage regimen. At the time of auto-HCT, 161 patients (39.7%) were in complete remission. The most frequent conditioning regimen was a carmustine, cytarabine, etoposide and melphalan regimen (BEAM) (n=373, 91.9%). The median time to neutrophil engraftment was 10 days (range: 9-20) and the median platelet engraftment time was 15 days (range: 9-74).The median number of lines post-HCT relapse was 1 (range: 0-6). Detailed characteristics are provided in Table 1.
HCT outcomes
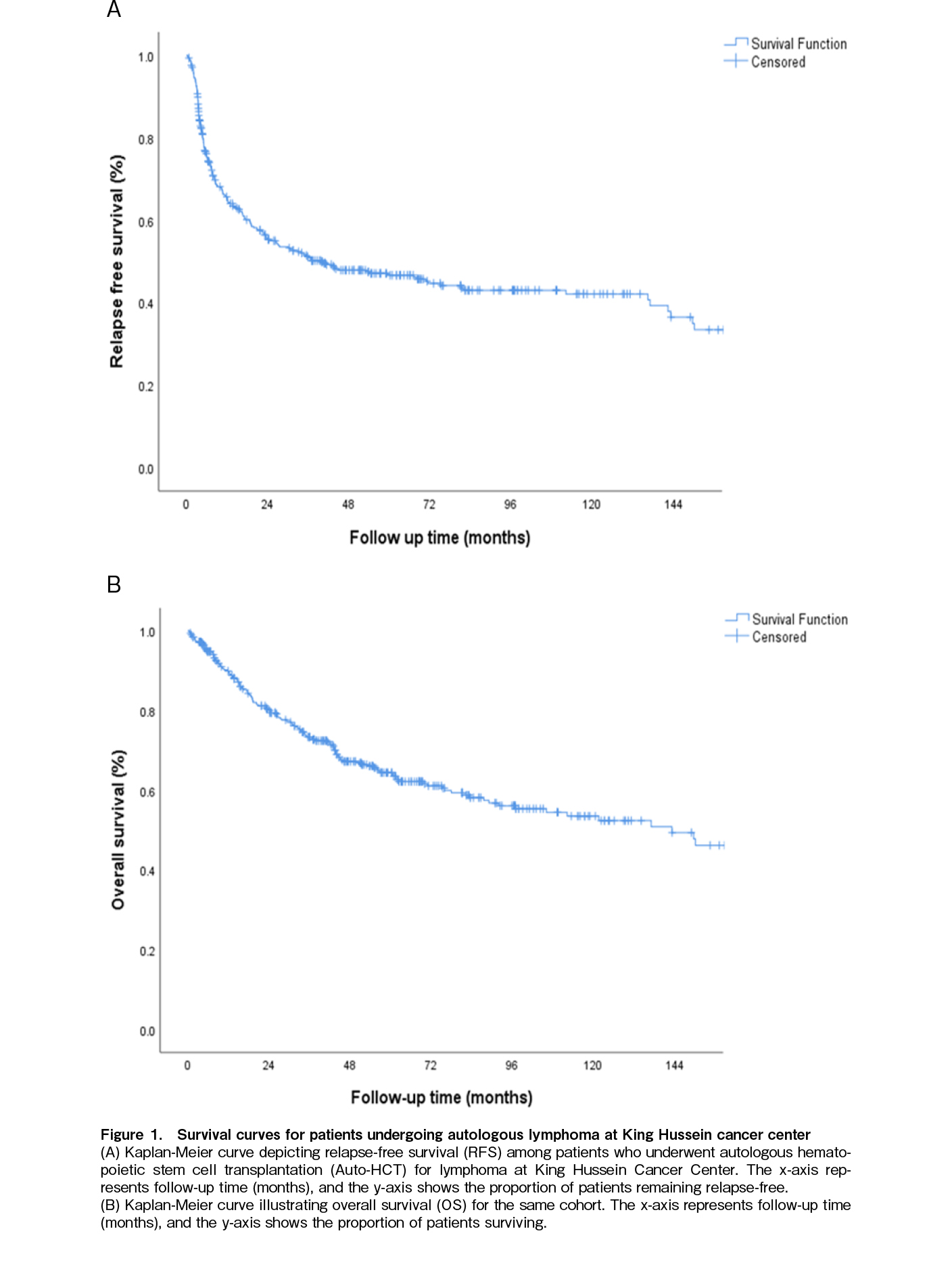
With a median follow-up of 43.4 months (range 0.1-242), The 5-year OS rate for the entire cohort was 64.4% (Figure 1A) and the median OS was not reached during the follow-up period. The median Relapse-free survival (RFS) was 21.9 months (range: 0.1-242.1) (Figure 1B). The median time to relapse post-HCT was 6.2 months (range: 1.1-144.4). Thirty-two patients (7.9%) experienced NRM during the study period, with t-MN accounting for 18.75% of these cases.
Characteristics and outcomes of patients with t-MN
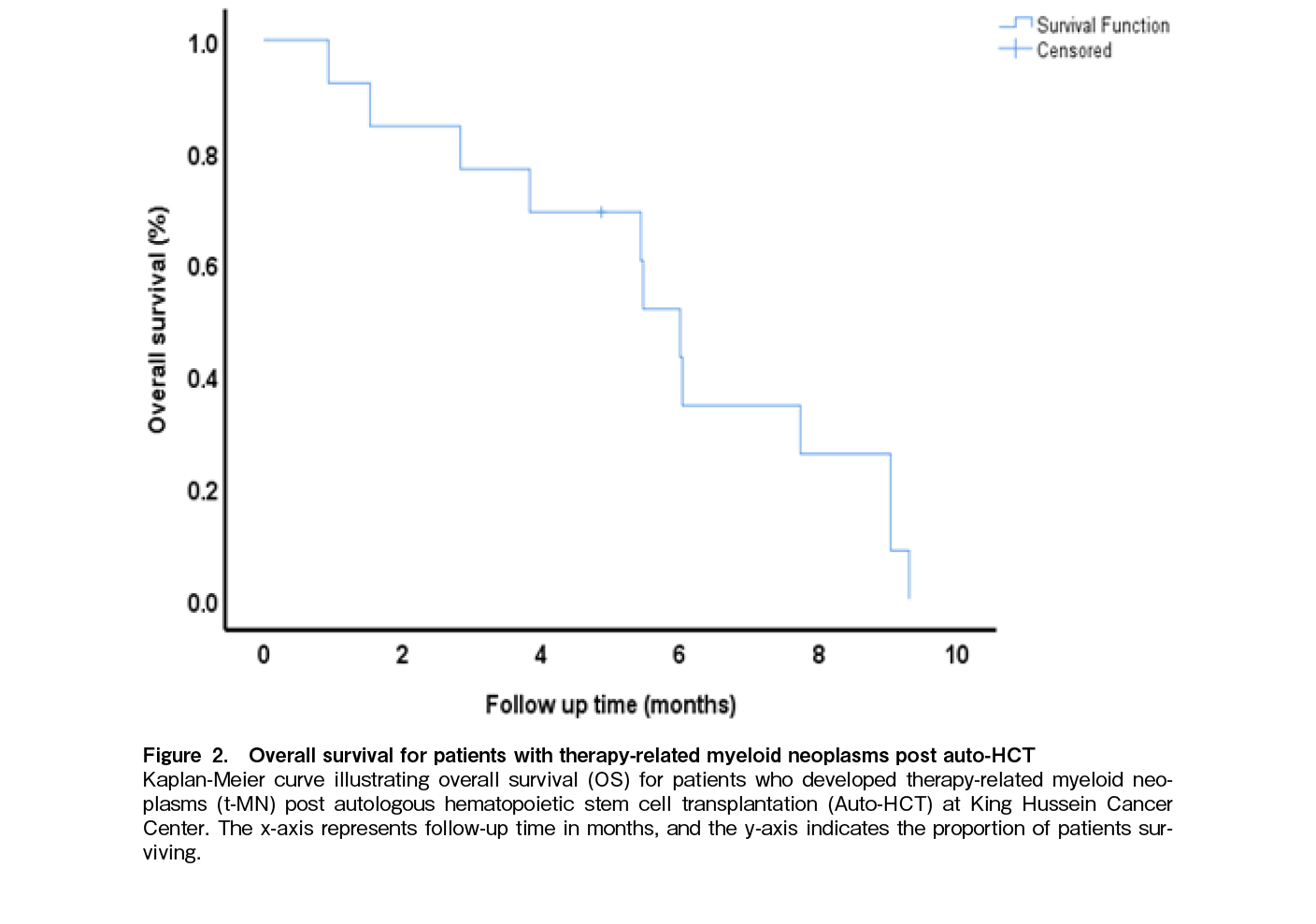
We identified 13 patients (3.2%), with a median time to diagnosis of 3.25 years (range 0.3-4.9) post-auto-HCT. Among these, 5 patients were diagnosed with AML, and 8 with MDS (Table 2). The median age at diagnosis of myeloid malignancy was 59 years (range: 34-69 years), while the median age at lymphoma diagnosis was 59 years (range: 34-69). There was a light male predominance at 54% (n=7). Regarding lymphoma subtypes, 5 patients had HL, and 8 had NHL. At the time of t-MN diagnosis, 6 patients (46%) were in remission, whereas 7 (54%) had relapsed disease. The median number of comorbidities was 1 (range: 0-4), and the median number of chemotherapy lines received prior to t-MN was 2 (range: 1-8). The median hemoglobin level was 8.6 g/dL (range: 6.7-10.9), median white blood cell count was 4.6 (range: 1.3-58.7) and median platelet count was 42 (range: 11-159). Karyotyping results showed complex abnormalities in 2 patients (15%), aneuploidy in 4 (31%) and inv(3) in 2 (15%). FISH analysis revealed del(7) in 6 patients (46%), del(5) in 4 (31%), trisomy 8 in 1 (7.5%), MLL rearrangement in 1 (7.5%), and normal findings in 4 (31%). NGS identified TP53 as the most common mutation at 15% (n=2) followed by ASXL1, TET2, DNMT3A, and PTPN11 in 1 patient each (7.5%). The median Revised International Prognostic Scoring System (R-IPSS) score for t-MDS was 4.5 (range: 4-9.5). Treatment for t-MN included azacitidine in 7 patients (54%), azacitidine plus venetoclax in 2 (15%), intensive induction in 1 (7.5%), allogeneic transplantation in 1 (7.5%), and best supportive care in 2 (15%). Response to t-MN treatment showed that 1 patient (7.5%) achieved complete remission (CR), while 10 patients (77%) had refractory disease. With a median follow-up post diagnosis of t-MN was 5.4 months (range: 0.9-102.2). The median overall survival post-t-MN diagnosis was 6 months (95% confidence interval (CI): 5-6.9), with a 1-year OS of 8% and a 3-year OS of 8% (Figure 2).
Predictors of t-MN development
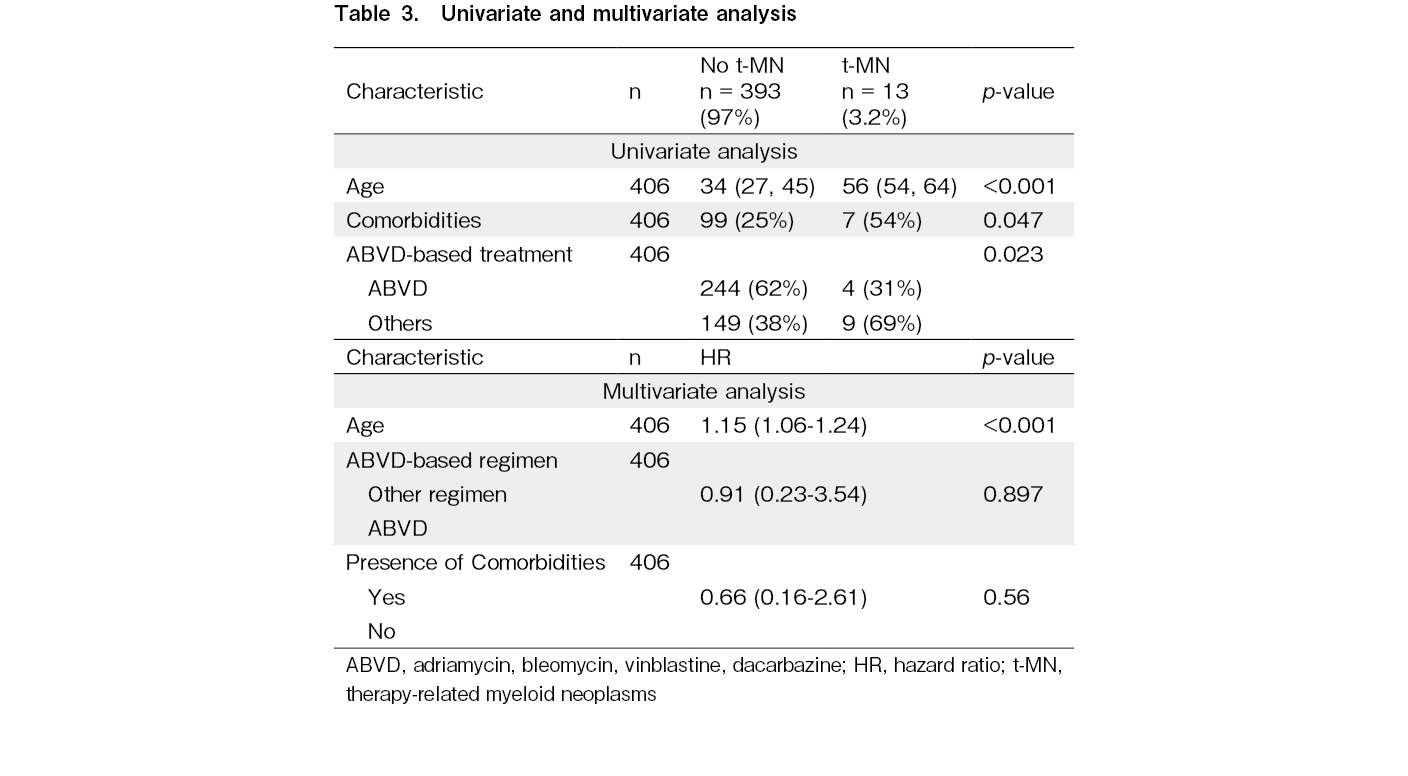
Patients who developed a t-MN were older (median age 55.7 vs 34 years; p < 0.001), more likely to have NHL (5.7% vs. 1.9%; p=0.039) and more likely to have comorbidities (54% vs. 25%; p=0.04).
First-line treatment also showed a statistically significant difference, with 69% of t-MN patients having received doxorubicin, bleomycin, vinblastine, dacarbazine (non-ABVD) therapies, compared to 38% of non-t-MN patients (p=0.023).
In the multivariate analysis, age was identified as the only significant independent predictor of t-MN development, with a p-value of < 0.001 (Table 3).
There was no association between gender, smoking status, site of involvement, stage, radiotherapy, response to first line treatment, number of salvage chemotherapy lines and conditioning regimen on the development of t-MN on univariate or multivariate analysis.
Discussion
The development of t-MN following high-dose chemotherapy and auto-HCT remains a serious complication and a leading cause of cumulative effects of chemotherapy, radiation, and high-dose conditioning regimens contribute to genomic instability, leading to clonal expansion and progression to secondary myeloid neoplasms13,14,20.
The heavy reliance on intensive chemotherapy in Jordan and other limited-resource settings due to lack of novel therapeutic options (e.g., checkpoint inhibitors, targeted therapies, bispecific T-cell engagers, and chimeric antigen T-cell therapy) results in patients receiving multiple cycles of chemotherapy in the second and third line (42% of patients received ≥ 2 salvage regimens) before proceeding to auto-HCT, further contributing to the risk of t-MN, however, despite this repeated exposure to intensive chemotherapy, the incidence of t-MN in our cohort appears to consistent with other published reports in the literature, signaling that there are potentially other factors contributing to the development of t-MN.
In our cohort, the overall incidence of t-MN was 3.2%, aligning with the lower range reported in the literature13,15. Age at the time of transplant emerged as the strongest predictor of t-MN, likely due to the accumulation of genomic or cytogenetic lesions, diminished hematopoietic regenerative capacity, and clonal evolution1,10,21. Notably, clonal hematopoiesis of indeterminate potential (CHIP) is increasingly recognized as a precursor to t-MN, particularly in older patients. A recent study by Yan et al. found CHIP in 14.3% of peripheral blood stem cell (PBSC) products, with TP53 mutations significantly increasing t-MN risk (adjusted hazard ratio [aHR] 4.50, 95% CI 1.54-13.19)22,23. A similar study by Gibson et al.48, utilized whole exome sequencing on pre-auto-HCT and t-MN samples from 12 patients who developed t-MN after auto-HCT for HL and NHL, in 6 of the 12 patients, the mutations found in the t-MN specimen were also detectable in the pre-auto-HCT specimen. Similarly, in separate cohort in the same study, a targeted sequencing on cryopreserved aliquots of autologous products from 401 patients who underwent auto-HCT for NHL, 120 patients (29.9%) had CHIP at the time of auto-HCT, which was associated with an increased rate of t-MN (10-year cumulative incidence, 14.1% v.s. 4.3% for those with and without CHIP, respectively; p=0.002). Patients with CHIP had significantly inferior OS compared with those without CHIP (10-year overall survival, 30.4% v.s. 60.9%, respectively; p < 0.001), including increased risk of death from t-MN and cardiovascular disease.
The cytogenetic abnormalities found included del(7), complex karyotypes, and chromosome 3 abnormalities were prevalent in our cohort with TP53 being the most common mutation found, all of which are well-known markers of poor prognosis which explains the dismal outcomes observed7,10,11,24. Abnormalities in chromosomes 5 and 7, often linked to prior exposure to alkylating agents, further emphasize the role of intensive chemotherapy in leukemogenesis.
BEAM, the most commonly used conditioning regimen in our cohort (91.9%), contains alkylating agents such as carmustine and melphalan, both associated with DNA damage and leukemogenesis, which in part explains the increased presence of del(7) and del(5) in our cohort. While BEAM remains the standard in many centers, including ours, its cumulative genotoxic effect may contribute to the development of t-MN. Alternative regimens such as lomustine, etoposide, cyclophosphamide, and dexamethasone (LEED) or thiotepa, etoposide, ara-C, melphalan (TEAM), which incorporate agents with potentially lower leukemogenic profiles, may be associated with a different risk profile. However, comparative data are limited and the risk of higher relapse rates with less intense regimens should be considered. Future studies exploring the impact of different conditioning regimens on secondary malignancies are warranted to inform safer protocols.
The median OS for t-MN patients was only six months, reflecting the aggressive nature of the disease is in the lower range of OS reported in the literature for t-MN, which have shown survival ranges of 8-14.5 months2,25,26. The majority of our patients received lower intensity treatment like azacitidine, and only 2 patients (15.3%) received newer options like azacitidine-venetoclax27, while allogeneic stem cell transplantation, the only curative option for t-MN, was rarely utilized in our cohort, likely due to advanced patient age and inadequate disease control as only 7.5% of patients achieved a CR28–30. Recently, novel therapies have emerged, including CPX-351, a liposomal formulation of cytarabine and daunorubicin, which has improved response rates and transplant outcomes by reducing early mortality31,32 and is now the standard of care for fit patients with secondary AML. Additional targeted approaches such as FLT3 and IDH1/2 inhibitors33, the venetoclax + hypomethylating agent (HMA) combination34,35, and experimental therapies like magrolimab (anti-CD47)36, eprenetapopt (APR246, anti-TP53)37, and MDM2 inhibitors (idasanutlin RG7388)38 offer potential avenues for improving survival in t-MN. However, all these strategies remain a bridge to allogeneic transplantation, which remains the definitive curative approach25,39. Given the strong association between older age and t-MN risk, and the dismal outcomes once t-MN develops, the role of auto-HCT in older patients should be carefully weighed, particularly in settings where novel therapies such as PD-1 inhibitors, antibody-drug conjugates, and bispecifics are accessible. In such cases, alternative treatment approaches may offer safer long-term outcomes. This underscores the need for individualized decision-making that incorporates patient age, comorbidities, and availability of newer agents.
The presence of cytogenetic abnormalities in stem cells suggests that pre-transplant screening using standard cytogenetics and FISH may help identify high-risk patients40–43. Emerging genetic testing methods, including microarray analysis, single nucleotide polymorphism studies, and metabolic profiling, hold promise for predicting t-MN susceptibility44–46. Prospective studies are needed to establish CHIP as a biomarker for risk stratification and to define genetic alterations that confer the highest risk.
Risk mitigation strategies should focus on modifying primary cancer treatment approaches, such as limiting alkylating agents or reducing radiation exposure in genetically predisposed patients. Screening for CHIP in autologous donors and reconsidering auto-HCT in CHIP-positive patients could further reduce the incidence of t-MN47–49. Finally, collaborative efforts across cancer centers are crucial for conducting prospective trials to refine our understanding of t-MN pathogenesis and develop evidence-based prevention strategies.
There are several limitations to our study. First, the retrospective nature of the data collection introduces the potential for missing information, particularly regarding prior treatments and comorbidities. Additionally, the relatively small number of t-MN cases limits the power of our analysis to detect risk factors and their interactions. Additionally, not all t-MN cases had comprehensive genetic testing, particularly in the earlier years of the study, due to introduction of molecular testing at our center in 2016. While this is consistent with the timing molecular testing became widespread in practice worldwide, it may have led to under detection of certain mutations such as TP53 compared to other cohort to t-MN in the literature.
Conclusion
t-MN are a major complication after auto-HCT in lymphoma patients, leading to poor survival and increased NRM. Our study identifies older age at transplantation as a key predictor of t-MN, suggesting age-related genetic vulnerabilities contribute to its development. The poor prognosis of t-MN underscores the need for enhanced surveillance, early diagnosis, and optimized treatment strategies. Vigilant post-transplant monitoring and further research into additional risk factors and preventive measures, such as pre-transplant clonal hematopoeisis screening, are essential to improving patient outcomes.
Author Contributions
AY: Conceptualization, Methodology, Data Curation, Writing – Original Draft, Visualization. AZ: Data Curation, Methodology. MR: Data Curation, Writing – Original Draft. AA: Writing – Original Draft. YT: Methodology, Writing – Review & Editing. LM: Writing – Review & Editing. WD: Writing – Review & Editing. OS: Writing – Review & Editing. KH: Writing – Review & Editing. KAL-R: Writing – Review & Editing. MM: Methodology, Formal Analysis, Writing – Review & Editing. ZAR: Conceptualization, Writing – Review & Editing, visualization, Supervision, Project Administration.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
1.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010; 363: 2091-101.
2.Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012; 18: 348-71.
3.Akhtari M, Bhatt VR, Tandra PK, Krishnamurthy J, Horstman H, Dreessen A, et al. Therapy-related myeloid neoplasms after autologous hematopoietic stem cell transplantation in lymphoma patients. Cancer Biol Ther. 2013; 14: 1077-88.
4.Gilliland DG, Gribben JG. Evaluation of the risk of therapy-related MDS/AML after autologous stem cell transplantation. Biol Blood Marrow Transplant. 2002; 8: 9-16.
5.Miller JS, Arthur DC, Litz CE, Neglia JP, Miller WJ, Weisdorf DJ. Myelodysplastic syndrome after autologous bone marrow transplantation: an additional late complication of curative cancer therapy. Blood. 1994; 83: 3780-6.
6.Brown JR, Yeckes H, Friedberg JW, Neuberg D, Kim H, Nadler LM, et al. Increasing incidence of late second malignancies after conditioning with cyclophosphamide and total-body irradiation and autologous bone marrow transplantation for non-hodgkin's lymphoma. J Clin Oncol. 2005; 23: 2208-14.
7.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz M, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127: 2391-405.
8.Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022; 36: 1703-19.
9.Voso MT, Falconi G, Fabiani E. What's new in the pathogenesis and treatment of therapy-related myeloid neoplasms. Blood. 2021; 138: 749-57.
10.Kuzmanovic T, Patel BJ, Sanikommu SR, Nagata Y, Awada H, Kerr CM, et al. Genomics of therapy-related myeloid neoplasms. Haematologica. 2020; 105: e98-101.
11.Wong TN, Ramsingh G, Young AL, Miller CA, Touma W, Welch JS, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2015; 518: 552-5.
12.Al Hamed R, Bazarbachi AH, Malard F, Harousseau JL, Mohty M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019; 9: 44.
13.Sevilla J, Rodríguez A, Hernández-Maraver D, de Bustos G, Aguado J, Ojeda E, et al. Secondary acute myeloid leukemia and myelodysplasia after autologous peripheral blood progenitor cell transplantation. Ann Hematol. 2002; 81: 11-5.
14.Nadiminti K, Sidiqi MH, Meleveedu K, Alkhateeb HB, Hogan WJ, Litzow M, et al. Characteristics and outcomes of therapy-related myeloid neoplasms following autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2021; 11: 63.
15.Darrington DL, Vose JM, Anderson JR, Bierman PJ, Bishop MR, Chan WC, et al. Incidence and characterization of secondary myelodysplastic syndrome and acute myelogenous leukemia following high-dose chemoradiotherapy and autologous stem-cell transplantation for lymphoid malignancies. J Clin Oncol. 1994; 12: 2527-34.
16.Traweek ST, Slovak ML, Nademanee AP, Brynes RK, Niland JC, Forman SJ. Clonal karyotypic hematopoietic cell abnormalities occurring after autologous bone marrow transplantation for Hodgkin's disease and non-Hodgkin's lymphoma. Blood. 1994; 84: 957-63.
17.Pedersen-Bjergaard J, Pedersen M, Myhre J, Geisler C. High risk of therapy-related leukemia after BEAM chemotherapy and autologous stem cell transplantation for previously treated lymphomas is mainly related to primary chemotherapy and not to the BEAM-transplantation procedure. Leukemia. 1997; 11: 1654-60.
18.Govindarajan R, Jagannath S, Flick JT, Vesole DH, Sawyer J, Barlogie B, et al. Preceding standard therapy is the likely cause of MDS after autotransplants for multiple myeloma. Br J Haematol. 1996; 95: 349-53.
19.Krishnan A, Bhatia S, Slovak ML, Arber DA, Niland JC, Nademanee A, et al. Predictors of therapy-related leukemia and myelodysplasia following autologous transplantation for lymphoma: an assessment of risk factors. Blood. 2000; 95: 1588-93.
20.Bhatia R, Van Heijzen K, Palmer A, Komiya A, Slovak ML, Chang KL, et al. Longitudinal assessment of hematopoietic abnormalities after autologous hematopoietic cell transplantation for lymphoma. J Clin Oncol. 2005; 23: 6699-711.
21.Mouhieddine TH, Sperling AS, Redd R, Park J, Leventhal M, Gibson CJ, et al. Clonal hematopoiesis is associated with adverse outcomes in multiple myeloma patients undergoing transplant. Nat Commun. 2020; 11: 2996.
22.Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015; 126: 9-16.
23.Yan C, Richard MA, Gibson CJ, He J, Bosworth A, Crossman DK, et al. Clonal Hematopoiesis and Therapy-Related Myeloid Neoplasms After Autologous Transplant for Hodgkin Lymphoma. J Clin Oncol. 2024; 42: 2415-24.
24.Lindsley RC, Saber W, Mar BG, Redd R, Wang T, Haagenson MD, et al. Prognostic Mutations in Myelodysplastic Syndrome after Stem-Cell Transplantation. N Engl J Med. 2017; 376: 536-47.
25.Fianchi L, Pagano L, Piciocchi A, Candoni A, Gaidano G, Breccia M, et al. Characteristics and outcome of therapy‐related myeloid neoplasms: Report from the I talian network on secondary leukemias. Am J Hematol. 2015; 90: E80-5.
26.Smith SM, Le Beau MM, Huo D, Karrison T, Sobecks RM, Anastasi J, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003; 102: 43-52.
27.DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med. 2020; 383: 617-29.
28.Finke J, Schmoor C, Bertz H, Marks R, Wäsch R, Zeiser R, et al. Long-term follow-up of therapy-related myelodysplasia and AML patients treated with allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2016; 51: 771-7.
29.Samra B, Richard-Carpentier G, Kadia TM, Ravandi F, Daver N, DiNardo CD, et al. Characteristics and outcomes of patients with therapy-related acute myeloid leukemia with normal karyotype. Blood Cancer J. 2020; 10: 47.
30.Granfeldt Østgård LS, Medeiros BC, Sengeløv H, Nørgaard M, Andersen MK, Dufva IH, et al. Epidemiology and Clinical Significance of Secondary and Therapy-Related Acute Myeloid Leukemia: A National Population-Based Cohort Study. J Clin Oncol. 2015; 33: 3641-9.
31.Lancet JE, Uy GL, Newell LF, Lin TL, Ritchie EK, Stuart RK, et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021; 8: e481-91.
32.Lin TL, Rizzieri DA, Ryan DH, Schiller GJ, Kolitz JE, Uy GL, et al. Older adults with newly diagnosed high-risk/secondary AML who achieved remission with CPX-351: phase 3 post hoc analyses. Blood Adv. 2021; 5: 1719-28.
33.Pullarkat VA, Levis M, Mannis GN, Strickland SA, Lin TL, Faderl S, et al. Preliminary Results By Age Group of Treatment with CPX-351 Plus Venetoclax in Adults with Newly Diagnosed AML: Subgroup Analysis of the V-FAST Phase 1b Master Trial. Blood. 2021; 138: 1268.
34.DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med. 2020; 383: 617-29.
35.Matthews AH, Perl AE, Luger SM, Loren AW, Gill SI, Porter DL, et al. Real-world effectiveness of CPX-351 vs venetoclax and azacitidine in acute myeloid leukemia. Blood Adv. 2022; 6: 3997-4005.
36.Chao MP, Takimoto CH, Feng DD, McKenna K, Gip P, Liu J, et al. Therapeutic Targeting of the Macrophage Immune Checkpoint CD47 in Myeloid Malignancies. Front Oncol. 2019; 9: 1380.
37.Cluzeau T, Sebert M, Rahmé R, Cuzzubbo S, Lehmann-Che J, Madelaine I, et al. Eprenetapopt Plus Azacitidine in TP53-Mutated Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Phase II Study by the Groupe Francophone des Myélodysplasies (GFM). J Clin Oncol. 2021; 39: 1575-83.
38.Khurana A, Shafer DA. MDM2 antagonists as a novel treatment option for acute myeloid leukemia: perspectives on the therapeutic potential of idasanutlin (RG7388). OncoTargets Ther. 2019; 12: 2903-10.
39.Kayser S, Döhner K, Krauter J, Köhne CH, Horst HA, Held G, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011; 117: 2137-45.
40.Del Cañizo M f, Amigo M f, Hernández JM, Sanz G, Núñez R, Carreras E, et al. Incidence and characterization of secondary myelodysplastic syndromes following autologous transplantation. Haematologica. 2000; 85: 403-9.
41.Chao NJ, Nademanee AP, Long GD, Schmidt GM, Donlon TA, Parker P, et al. Importance of bone marrow cytogenetic evaluation before autologous bone marrow transplantation for Hodgkin's disease. J Clin Oncol. 1991; 9: 1575-9.
42.Lillington DM, Micallef INM, Carpenter E, Neat MJ, Amess JAL, Matthews J, et al. Genetic susceptibility to Hodgkin's disease and secondary neoplasias: FISH analysis reveals patients at high risk of developing secondary neoplasia. Ann Oncol. 2002; 13: 40-3.
43.Cachia PG, Culligan DJ, Clark RE, Whittaker JA, Jacobs A, Padua RA. Clonal haemopoiesis following cytotoxic therapy for lymphoma. Leukemia. 1993; 7: 795-800.
44.Ding Y, Sun CL, Li L, Li M, Francisco L, Sabado M, et al. Genetic susceptibility to therapy-related leukemia after Hodgkin lymphoma or non-Hodgkin lymphoma: role of drug metabolism, apoptosis and DNA repair. Blood Cancer J. 2012; 2: e58.
45.Cano KE, Li L, Bhatia S, Bhatia R, Forman SJ, Chen Y. NMR-based metabolomic analysis of the molecular pathogenesis of therapy-related myelodysplasia/acute myeloid leukemia. J Proteome Res. 2011; 10: 2873-81.
46.Li L, Li M, Sun C, Francisco L, Chakraborty S, Sabado M, et al. Altered hematopoietic cell gene expression precedes development of therapy-related myelodysplasia/acute myeloid leukemia and identifies patients at risk. Cancer Cell. 2011; 20: 591-605.
47.Rojek K, Nickels E, Neistadt B, Marquez R, Wickrema A, Artz A, et al. Identifying Inherited and Acquired Genetic Factors Involved in Poor Stem Cell Mobilization and Donor-Derived Malignancy. Biol Blood Marrow Transplant. 2016; 22: 2100-3.
48.Gibson CJ, Lindsley RC, Tchekmedyian V, Mar BG, Shi J, Jaiswal S, et al. Clonal Hematopoiesis Associated With Adverse Outcomes After Autologous Stem-Cell Transplantation for Lymphoma. J Clin Oncol. 2017; 35: 1598-1605.
49.McNerney ME, Godley LA, Le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 2017; 17: 513-27.
Search
News