Volume 8 (2025) Issue 3 No.2 Pages 225-227
Abstract
Several reports have been published on autoimmune hematologic complications, including immune thrombocytopenia (ITP), after cord blood transplantation (CBT). However, there have been no reports of late-onset ITP following CBT. A 51-year-old male with chronic myelomonocytic leukemia received unrelated CBT in 2012. During regular follow-up visits every 3-6 months, his complete blood count remained normal until March 2024. In September 2024, 12 years after the CBT, the patient suddenly developed severe thrombocytopenia. Following a diagnosis of ITP, the patient was treated with intravenous immunoglobulins and prednisone. With follow-up period of 5 months after the onset of ITP, the patient is still alive with a platelet count of 126 × 109/L. This case suggests that late-onset ITP after CBT occurred suddenly and may be life-threatening. Long-term follow-ups and regular clinic visits may reduce the risk of delays in diagnosis and treatment.
Introduction
Although the incidence of immune thrombocytopenia (ITP) after allogeneic stem cell transplantation (SCT) is relatively low (0.9-1.4%), it is a well-known complication of SCT1. Recently, in Europe, the use of unrelated donors and haploidentical donors has increased, while the use of cord blood (CB) has decreased2. In Japan, however, CB remains a major alternative stem cell source for patients without HLA-compatible related or unrelated donors. More than 1,300 single-unit CB transplantations (CBT) are performed in Japan annually, with the total number of CBTs reaching 25,100 in 20243. Several reports of autoimmune hematologic complications, including ITP, after CBT have been published4–7. However, the incidence and outcomes of ITP after CBT were not fully described. We report here a patient who developed ITP 12 years after CBT and was successfully treated with intravenous immunoglobulin (IVIg) and corticosteroids.
Case Presentation
A 51-year-old male with chronic myelomonocytic leukemia (CMML) with trisomy 8 received his first unrelated CBT in September 2012. The conditioning regimen included 12 Gy of total body irradiation, granulocyte colony-stimulating factor combined with 12 g/m2 cytarabine, and 120 mg/kg cyclophosphamide8. The patient received standard cyclosporine and methotrexate as a graft-versus-host disease (GVHD) prophylaxis. Primary graft failure was confirmed at 29 days, and the patient received a second CBT (CBT2) at 36 days after the first CBT. The conditioning regimen for CBT2 included 120 mg/m2 (40 mg/m2, 3 days) fludarabine. An absolute neutrophil counts greater than 0.5 × 109/L was achieved on day 25 after CBT2. Chimerism evaluation using short tandem repeats (STR) revealed complete donor chimerism of bone marrow (BM) cells on day 38 after CBT2. During the follow-up period after CBT2, overall grade I (skin stage 2) acute GVHD and limited (skin alone) NIH mild chronic GVHD were observed. Immunosuppressive treatment was discontinued 111 days after CBT2. During regular follow-up visits every 3-6 months, his complete blood count remained normal until March 2024. In August 2024, the patient developed acute bronchitis, which improved and both influenza virus and severe acute respiratory disease coronavirus 2 were not detected. In September 2024, 12 years (4,381 days) after CBT2, the patient suddenly developed severe thrombocytopenia without active viral infection. Peripheral blood analysis showed thrombocytopenia with a platelet count of 19 × 109/L. Thereafter, it decreased to 3 × 109/L. BM examination showed normal megakaryocytes with no evidence of increased abnormal blast cells or dysplastic changes. Cytogenetic analysis of BM cells revealed a normal karyotype of 46,XY. Chimerism evaluation using STR revealed complete donor chimerism of BM cells. The platelet-associated immunoglobulin G (PA-IgG) and the immature platelet fraction (IPF) of peripheral blood were increased. PA-IgG was 3,540 ng/107 cells and IPF was 29.6%. After the diagnosis of ITP, the patient was initially treated with IVIg (400 mg/kg/day for 5 days) and prednisone (0.5 mg/kg/day). The patient achieved a sustained complete response (CR) with a platelet count of > 100 × 109/L after 35 days. With follow-up period of 5 months after the onset of ITP, the patient is still alive with a platelet count of 126 × 109/L.
Discussion
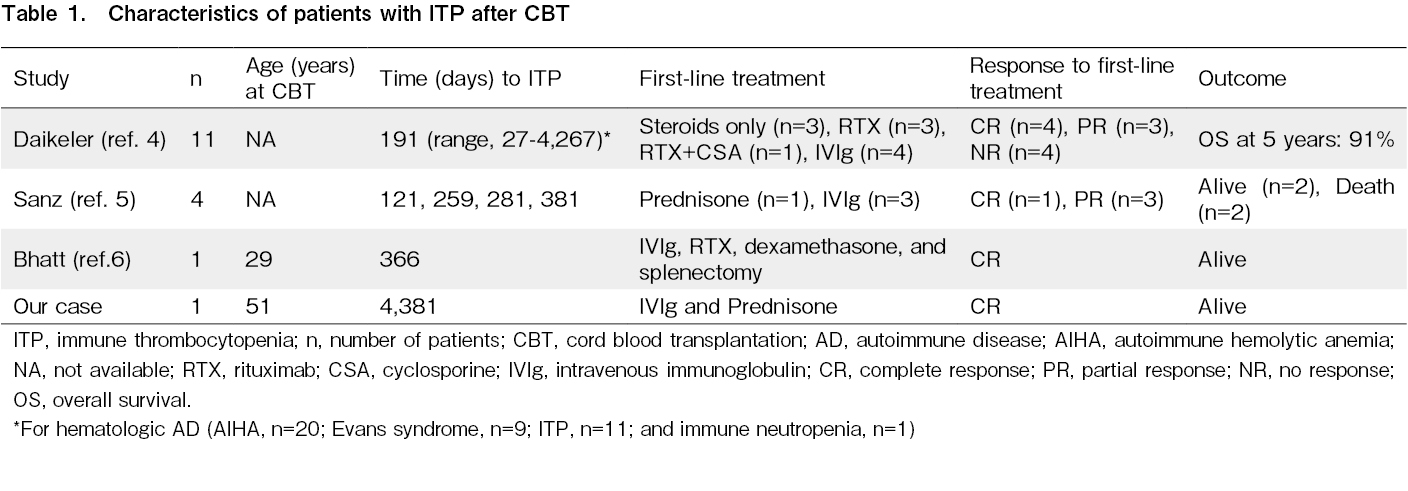
Only a few patients with ITP after CBT have been reported till now (Table 1)4–6. The result of EUROCORD and the European Society for Blood and Marrow Transplantation (EBMT) registry revealed that 52 out of 778 patients had autoimmune diseases (AD), including ITP (Daikeler et al., 2013)4. The cumulative incidence (CI) of AD after CBT was 5% after 1 year and 6.6% after 5 years. Among the 52 patients, 41 had hematologic AD (autoimmune hemolytic anemia [AIHA], n = 20; Evans syndrome, n = 9; ITP, n = 11; and immune neutropenia, n = 1). The median time to the occurrence of hematologic AD was 191 days (range 27-4,267) after CBT. The risk factor for developing AD was nonmalignant disease, which is an indication of CBT. Patients with ITP and Evans syndrome were mostly treated with steroids, rituximab, and cyclosporine. The overall survival at 5 years was 91% for patients who developed ITP. The authors concluded that potentially life-threatening AD may follow CBT. Since AIHA and ITP were most commonly observed in such cases, they must be considered as possible reasons for unexplained cytopenia after CBT. In 2014, Sanz et al. reported a single-center experience of autoimmune cytopenia after CBT in adults with hematologic malignancies5. Among the 281 patients, four developed ITP between 121 and 381 days after CBT. CI for ITP at 3 years was 1.4%. One patient who received only prednisone achieved sustained CR. Three patients who received first-line IVIg therapy achieved a temporary response. Of the three patients, one received prednisone, achieving CR. The remaining two patients received rituximab; one achieved CR, and the other achieved a response. Among the four patients, two patients survived, and the remaining two patients died of complications associated with chronic GVHD and leukemia relapse. In 2016, Bhatt et al. conducted a single-center retrospective study on autoimmune cytopenia after CBT6. Among 152 patients, 10 developed autoimmune cytopenia (AIHA, n = 8; ITP, n = 1; and both AIHA and ITP, n = 1). One patient developed ITP 12.2 months after CBT, and one developed both AIHA and ITP 5.8 months after CBT. The patient who developed ITP only received IVIg, rituximab, dexamethasone, romiplostim, and splenectomy, achieving CR. The patient who developed both AIHA and ITP received IVIg, rituximab, and methylprednisolone, achieving CR. Both patients are alive and disease-free.
Although the occurrence of autoimmune cytopenia after CBT is rare, it is potentially life-threatening. Our patient developed ITP 12 years (4,381 days) after CBT, which, to our knowledge, is the slowest onset of ITP after CBT. Long-term follow-ups and regular clinic visits may reduce the risk of delays in diagnosis and treatment of autoimmune cytopenia after CBT.
Author Contributions
SA, KM, HT and JO wrote the manuscript and treated the patient. SK and TK wrote the manuscript and collected data. All authors contributed to the final version of the manuscript and approved it for publication.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Li Z, Rubinstein SM, Thota R, Savani M, Brissot E, Shaw BE, et al. Immune-Mediated Complications after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2016; 22: 1368-75.
2.Passweg JR, Baldomero H, Atlija M, Kleovoulou I, Witaszek A, Alexander T, et al. The 2023 EBMT report on hematopoietic cell transplantation and cellular therapies. Increased use of allogeneic HCT for myeloid malignancies and of CAR-T at the expense of autologous HCT. Bone Marrow Transplant. 2025; 60: 519-28.
3.Japanese Red Cross Society. https://www.bs.jrc.or.jp/bmdc/cordblooddonor/image/1-6_01.png [Accessed: 15 February 2025]
4.Daikeler T, Labopin M, Ruggeri A, Crotta A, Abinun M, Hussein AA, et al. New autoimmune diseases after cord blood transplantation: a retrospective study of EUROCORD and the Autoimmune Disease Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2013; 121: 1059-64.
5.Sanz J, Arango M, Carpio N, Montesinos P, Moscardó F, Martín G, et al. Autoimmune cytopenias after umbilical cord blood transplantation in adults with hematological malignancies: a single-center experience. Bone Marrow Transplant. 2014; 49: 1084-8.
6.Bhatt V, Shune L, Lauer E, Lubin M, Devlin SM, Scaradavou A, et al. Autoimmune hemolysis and immune thrombocytopenic purpura after cord blood transplantation may be life-threatening and warrants early therapy with rituximab. Bone Marrow Transplant. 2016; 51: 1579-83.
7.Ibrahim U, Keyzner A. Autoimmune hematologic complications of umbilical cord blood transplantation. Hematol Oncol Stem Cell Ther. 2021; 14: 104-9.
8.Konuma T, Ooi J, Nagayama H, Tomonari A, Tsukada N, Kato S, et al. Long-term outcomes following the addition of granulocyte colony-stimulating factor-combined high-dose cytarabine to total body irradiation and cyclophosphamide conditioning in single-unit cord blood transplantation for myeloid malignancies. Ann Hematol. 2022; 101: 177-89.
Search
News