Volume 8 (2025) Issue 4 No.5 Pages 281-285
Abstract
Allogeneic stem cell transplantation from an HLA-mismatched unrelated donor was performed for a patient with leukocyte adhesion deficiency type III with a myeloablative regimen including full-dose busulfan. Mixed chimerism with donor-derived T cells at less than 10% was observed within 4 weeks after transplantation. Repeated cycles of discontinuation and resumption of tacrolimus early after transplantation were performed with the aim of reversing the recipient-dominant T-cell chimerism. Specifically, tacrolimus was quickly tapered on day 15 and discontinued on day 20 when the recipient's chimerism increased, and resumed upon the observation of early signs of acute graft-versus-host disease, such as fever and skin rash, on day 24. This process was repeated from day 30 to day 44. All subsets, including granulocytes, T cells, and natural killer cells, attained donor chimerism of more than 90% on day 42 after transplantation and 100% at day 82 and beyond. Immunosuppressant dosage adjustments may be a treatment option for mixed chimerism after stem cell transplantation.
Introduction
Leukocyte adhesion deficiency type III (LADIII) is an extremely rare primary immunodeficiency disease caused by a defect in leukocyte integrin activation associated with a mutation in the FERMT3 gene. This gene encodes the fermitin family homolog 3 (FERMT3) protein, also known as kindlin-3 (KIND3), MIG2-like protein (MIG2B), and unc-112-related protein 2 (URP2). LADIII is characterized by leukocyte adhesion molecule abnormalities and platelet dysfunction1. Its prognosis is dismal because of recurrent infections and a tendency for increased bleeding. Hematopoietic stem cell transplantation (HSCT) is considered to be the only treatment for LADIII. However, HSCT for LADIII has been associated with an increased incidence of graft failure (GF) and hepatic sinusoidal obstruction syndrome, especially in patients complicated with osteopetrosis2–4. Mixed chimerism followed by late GF is frequently observed, especially in alternative donor transplants. We attempted repeated cycles of discontinuation and resumption of tacrolimus early after transplantation to reverse highly recipient-dominant T-cell mixed chimerism that emerged after human leukocyte antigen (HLA)-mismatched unrelated bone marrow transplantation (UBMT) in a patient with LADIII with osteopetrosis.
Case Presentation
The female patient, the first child of consanguineous parents who were first cousins, developed subcutaneous bleeding in the trunk and extremities at birth after 39 weeks of gestation. Blood examination revealed a leukocyte count of 42,240/μL, hemoglobin of 12.8 g/dL, and a platelet count of 15.0 × 104/μL. Anemia with 5.3 g/dL of hemoglobin was observed, and a red blood cell transfusion was performed 17 days after birth. Periumbilical cellulitis with fever developed 20 days after birth, which improved with antibiotics. However, leukocytosis of around 30,000 to 50,000/μL persisted, and slight trauma repeatedly led to both subcutaneous and gingival bleeding. The patient was referred to Tokai University Hospital when she was 10 months old. Hematological findings at admission were as follows: leukocyte count of 31,000/μL, hemoglobin of 10.7 g/dL, platelet count of 21.5 × 104/μL, and CRP of 0.33 mg/dL. Bone marrow showed mild hyperplasia. Before admission, neither severe infection nor life-threatening hemorrhage was observed, and neither prophylactic antibiotics nor platelet transfusions were administered.
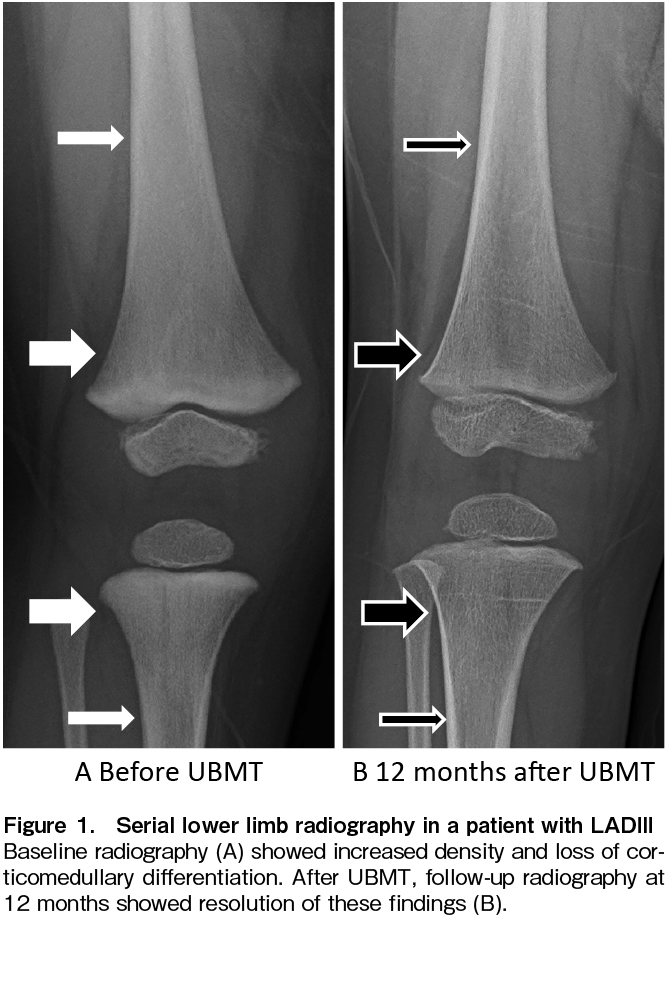
LADIII was diagnosed as follows: Neutrophils and monocytes were stimulated with fMLP and PMA (PKS analog) to demonstrate inducible defects in β2 integrin. Western blotting revealed defective kindlin-3 expression in platelets. Genetic analysis detected the homozygous variant kindlin-3 c.918 G>A, p277 Trp>stop. A vector expressing this kindlin-3 variant was transfected into 293T cells and it was confirmed to be responsible for the defective kindlin-3 expression. Before transplantation, the metaphysis of the femur and tibia showed mild sclerosis, increased radiodensity, and absence of the trabecular pattern (thick arrows, Figure 1A); corticomedullary differentiation was diminished in the diaphysis (thin arrows, Figure 1A). These findings reflected the complications of osteopetrosis.
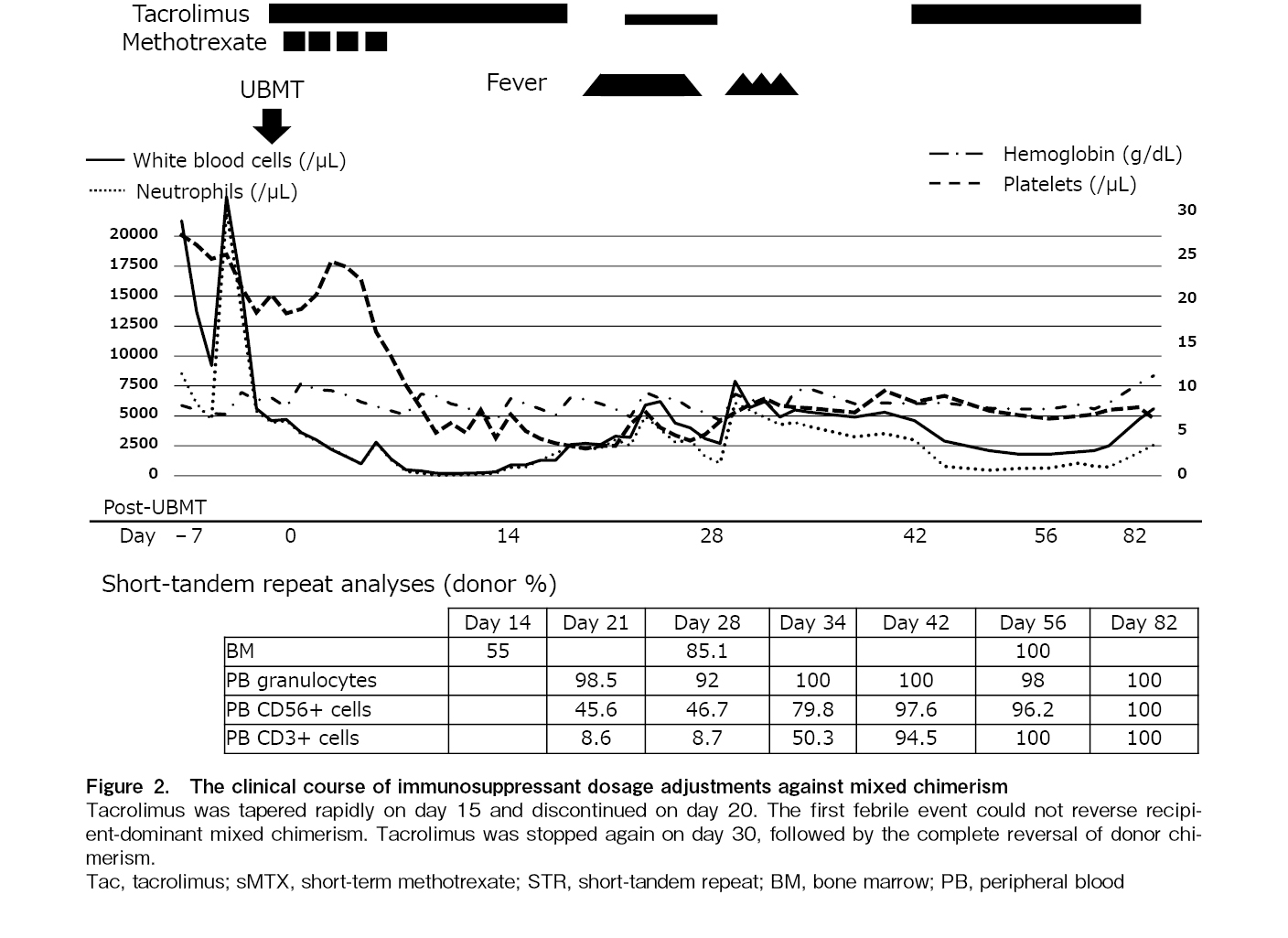
The patient underwent UBMT from a donor with HLA-DRB1 mismatched in the rejection direction at the age of 1 year and 7 months after written informed consent was obtained from the patient's parents (Figure 2). The conditioning procedure comprised 30 mg/m2 fludarabine once daily for 6 days (days −7 to −2), 0.8 mg/kg intravenous busulfan four times a day for 4 days (days −5 to −2), and 1.25 mg/kg anti-thymocyte globulin (ATG) daily for 4 days (days −5 to −2). Prophylaxis against graft-versus-host disease (GVHD) was performed by the short-term administration of methotrexate (0.5 mg/kg/day on post-transplantation day 1 and 0.35 mg/kg/day on days 3, 6, and 11) and tacrolimus (0.03 mg/kg/day continuous intravenous infusion). Dalteparin sodium was administered at 75 IU/kg daily as prophylaxis for hepatic sinusoidal obstruction syndrome. Chimerism analysis was performed using the short-tandem repeat (STR) method. Samples were bone marrow (BM) at 2, 4, and 8 weeks post-transplantation and peripheral blood (PB), divided into granulocytes, T lymphocytes, and NK cells, at 4, 8, and 14 weeks post-transplantation. Additional tests were performed as appropriate in the presence of progressive mixed chimerism.
On day 0, 4.3 × 108/kg of BM nucleated cells (2.5 × 106/kg of CD34-positive cells) were infused. Granulocyte colony-stimulating factor at a dose of 5 μg/kg was administered from day 5 post-transplantation until a neutrophil count > 1.5 × 109/L was achieved. A neutrophil count > 0.5 × 109/L and a platelet count > 20 × 109/L were obtained on days 15 and 28, respectively.
Donor cell engraftment was examined by the STR method on day 14, and 55.0% of the donor-type cells were detected by using BM cells, while only 5.9% and 20.8% were detected by using PB T cells and NK cells, respectively. To activate donor-derived T cells against imminent graft rejection, tacrolimus was reduced to one-third of the starting dose on day 15 and discontinued on day 19 after confirming no response in clinical symptoms upon tapering. A fever of 38.1℃ appeared on day 20 and intensified to 39.9℃ on day 23 with skin flushing but without fixed maculopapular rash. Tacrolimus was restarted at 0.015 mg/kg/day on day 24. The fever resolved on day 27. However, donor T cells remained at 8.7% in PB on day 28, and tacrolimus was stopped again on day 30. Fever as high as 38.9℃ developed again with mild skin eruption, and donor cells increased to 50.3% in T cells, 79.8% in NK cells, and 100% in granulocytes on day 34. The donor chimerism in BM increased to 85.1% on day 28 and reached 100% on day 56. From day 82 until 6.5 years after UBMT, all chimeric studies using PB samples, separated into granulocytes, T cells, and NK cells, consistently showed 100% donor-derived cells. No serious complications such as infections, bleeding, or GVHD were observed. Serial skeletal X-ray studies revealed a reversal of generalized bony sclerosis, probably due to the remodeling process without new sclerotic bone formation (Figure 1B). For 6.5 years after UBMT, the patient has been free of infection and bleeding with normal growth and development.
Discussion
HSCT has been applied in most patients with LADIII. However, the transplant-related mortality rose to approximately 20% of recipients5. Stepensky et al.2 treated three patients with LADIII by HSCT from an HLA-identical sibling donor (one patient) or alternative donor (two patients), and only one patient who received matched sibling bone marrow showed an uneventful clinical course. Regarding the two alternative donor transplants, one patient died of infection and the other suffered from graft rejection followed by successful re-HSCT. Elhasid et al.3 reported prompt recovery of recipient hematopoiesis after two consecutive haploidentical HSCTs from the mother with two different conditioning regimens, including a myeloablative one. Thus, GF is the first significant obstacle to have been overcome in HSCT for LADIII.
LADIII has been reported to be accompanied by osteopetrosis in 32% of patients6, which has been shown to make HSCT more challenging via an increase in the incidence of transplant-related morbidities such as GF. The 5-year survival rates among recipients with infantile osteopetrosis were 62% after HLA-matched sibling transplantation and 42% after alternative donor transplantation. GF was the most common cause of death, accounting for 50% of deaths after HLA-matched sibling transplantation and 43% of those after alternative donor transplantation7. Osteopetrosis associated with LADIII has also been shown to improve after HSCT2 and, in the present case, the radiographic findings confirmed such improvement (Figure 1B).
Reduced-intensity conditioning (RIC) has been considered preferable for growth and development after HSCT in younger patients with non-malignant diseases. Although some LADIII patients achieved sustained engraftment after HSCT using an RIC regimen, those successes were limited to within HLA-identical sibling transplants9. Orchard et al.7 reported that the use of an RIC regimen in patients with osteopetrosis to lower transplant-related morbidity resulted in high rates of GF and death in alternative donor transplants.
Only a limited number of papers on HSCT for LADIII detailed the conditioning regimen used. For example, Bakhtiar et al. reported that 11 patients underwent HSCT with myeloablative conditioning, but the 3-year event-free survival rate was only 56%5. Two patients died of either GF followed by infection or chronic GVHD. Compared with HSCT for LADIII, many more cases of HSCT for osteopetrosis have been reported, and the optimal conditioning regimen has been discussed. Natsheh et al. reported an excellent survival rate of 96% after HSCT for infantile malignant osteopetrosis conditioned with a fludarabine-based regimen8. The most commonly used regimen in the fludarabine group was a combination of fludarabine, busulfan, and ATG. ATG included in the conditioning regimen induces in vivo T-lymphocyte depletion and effectively prevents GVHD in unrelated donor transplants. Based on the above, we selected myeloablative conditioning with these three drugs in this case.
Most LADIII patients have leukocytosis with variable findings of both erythropoiesis and thrombopoiesis. It has been suggested that the phenomenon of hyperleukocytosis in LADIII is not only a consequence of impaired neutrophil migration but also the result of an inflammatory process following the dysregulation of cytokines10. This condition may be one of the explanations for the highly recipient-dominant T-cell mixed chimerism that was observed early after HSCT in our patient. Thus, additional intensification of conditioning may not be sufficient to obtain durable engraftment in LADIII patients, and an approach consisting of rapid tapering and discontinuation of tacrolimus without any target value, followed by resumption early after transplantation that facilitates donor-cell engraftment, should be considered. Kojima et al.11 showed that rapid tapering and cessation of calcineurin inhibitors brought about complete reversal of donor chimerism and simultaneously improved concomitant autoimmune disease. Another critical point in this approach is not to overlook the optimal timing of the intervention to prevent imminent GF. GF advances without any signs such as fever, regardless of disease. Only serial quantitative chimerism analyses make it possible to determine the appropriate timing of repeated cycles of discontinuation and resumption of tacrolimus early after transplantation. Concern about the induction of GVHD is unnecessary because T-cell mixed chimerism is significantly associated with decreased risks of moderate-to-severe acute GVHD and death due to GVHD12.
In summary, repeated cycles of discontinuation and resumption of tacrolimus even early after transplantation, such as within 30 days, might be considered in accordance with the emergence of mixed chimerism in HSCT for LADIII. Serial quantitative chimerism analyses are helpful in detecting mixed chimerism at an early stage because GF always develops and progresses in a clinically asymptomatic manner.
Acknowledgments
We are grateful to Dr. Hirokazu Kashiwagi (Osaka University) for help with the diagnostic process and Dr. Yasuhiko Ito (Nagoya City University) for medical support before HSCT.
Author Contributions
TK and HY designed the research and wrote the manuscript, while ES, KO, SO, YF, and HH performed clinical care. All authors approved the final version of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Kuijpers TW, van de Vijver E, Weterman MA, de Boer M, Tool AT, van den Berg TK, et al. LAD-1/variant syndrome is caused by mutations in FERMT3. Blood. 2009; 113: 4740-6.
2.Stepensky PY, Wolach B, Gavrieli R, Rousso S, Ami TB, Goldman V, et al. Leukocyte adhesion deficiency type III: clinical features and treatment with stem cell transplantation. J Pediatr Hematol Oncol. 2015; 37: 264-8.
3.Elhasid R, Kilic SS, Ben-Arush M, Etzioni A, Rowe JM. Prompt recovery of recipient hematopoiesis after two consecutive haploidentical peripheral blood SCTs in a child with leukocyte adhesion defect III syndrome. Bone Marrow Transplant. 2010; 45: 413-4.
4.Crazzolara R, Maurer K, Schulze H, Zieger B, Zustin J, Schulz AS. A new mutation in the KINDLIN-3 gene ablates integrin-dependent leukocyte, platelet, and osteoclast function in a patient with leukocyte adhesion deficiency-III. Pediatr Blood Cancer. 2015; 62: 1677-9.
5.Bakhtiar S, Salzmann-Manrique E, Blok HJ, Eikema DJ, Hazelaar S, Ayas M, et al. Allogeneic hematopoietic stem cell transplantation in leukocyte adhesion deficiency type I and III. Blood Adv. 2021; 5: 262-3.
6.Saultier P, Szepetowski S, Canault M, Falaise C, Poggi M, Suchon P, et al. Long-term management of leukocyte adhesion deficiency type III without hematopoietic stem cell transplantation. Haematologica. 2018; 103: e264-7.
7.Orchard PJ, Fasth AL, Le Rademacher J, He W, Boelens JJ, Horwitz EM, et al. Hematopoietic stem cell transplantation for infantile osteopetrosis. Blood. 2015; 126: 270-6.
8.Natsheh J, Drozdinsky G, Simanovsky N, Lamdan R, Erlich O, Gorelik N, et al. Improved Outcomes of Hematopoietic Stem Cell Transplantation in Patients With Infantile Malignant Osteopetrosis Using Fludarabine-Based Conditioning. Pediatr Blood Cancer. 2016; 63: 535-40.
9.Barhoom D, Behfar M, Mohseni R, Hamidieh AA. Successful allogeneic stem cell transplantation with a reduced-intensity conditioning in a case of leukocyte adhesion deficiency type III. Hematol Transfus Cell Ther. 2024; 46: 300-2.
10.Moutsopoulos NM, Konkel J, Sarmadi M, Eskan MA, Wild T, Dutzan N, et al. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci Transl Med. 2014; 6: 229ra40.
11.Kojima R, Kami M, Kim SW, Murashige N, Kishi Y, Hori A, et al. Induction of graft-versus-autoimmune (GVA) disease effect against refractory psoriasis by complete donor-type chimerism and graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003; 32: 439-42.
12.Mattsson J, Uzunel M, Remberger M, Ringdén O. T cell mixed chimerism is significantly correlated to a decreased risk of acute graft-versus-host disease after allogeneic stem cell transplantation. Transplantation. 2001; 71: 433-9.
Search
News