Volume 8 (2025) Issue 3 No.4 Pages 234-243
Abstract
Background: Bone marrow (BM) Measurable Residual Disease (MRD) assessments underestimate disease burden in multiple myeloma, as focal lesions can exist outside the marrow. Functional imaging, like positron emission tomography-computed tomography (PET-CT), offers valuable insights into residual disease beyond the marrow. Combining marrow flow cytometry (FCM) with PET-CT for a composite MRD (cMRD) assessment before and after autologous stem cell transplant (ASCT) is expected to provide prognostic information, particularly in settings where patients receive extended duration of anti-myeloma therapy prior to ASCT.
Methods: In this retrospective cohort study, we evaluated the prognostic impact of cMRD in newly diagnosed multiple myeloma (NDMM) patients who underwent triplet/quadruplet-based induction followed by ASCT from January 2017 to June 2023. cMRD was assessed before ASCT and again around day 100 post-transplant. cMRD negativity was defined as undetectable residual clonal plasma cells (sensitivity 1×10-5) on multi coloured FCM and PET-CT negativity per The International Myeloma Working Group criteria.
Results: Among 106 patients undergoing ASCT, 82 had cMRD assessments before and on day 100 post-ASCT. Median pre-ASCT treatment duration was 11 months (interquartile range [IQR]: 4-18). At the pre-ASCT time point, sixty seven percent patients were bone marrow MRD negative (BM-MRDPRE-), while 38% were PET-CT negative (PETPRE-). Post-ASCT, these rates were 74% (BM-MRDPOST-) and 49% (PET-CTPOST-) respectively. At a median follow-up of 35 months (IQR: 23.5-58), median time to next treatment (TTNT) and overall survival (OS) were not reached. At three years, TTNT was significantly higher in patients who were cMRD-negative before ASCT compared to those who were cMRD-positive [91% (confidence interval (CI): 77-100) versus 67% (CI: 52-80); p=0.027]. BM-MRDPRE- and PETPRE- were both independently associated with improved TTNT on univariate analysis [Hazard Ratio: 0.32 (0.14-0.74) and 0.45 (0.23-0.94) respectively]. Post-ASCT cMRD status did not significantly impact TTNT [82% (CI: 68-96) versus 65% (CI: 51-69); p=0.116]. Three-year TTNT rates were similar among patients with and without baseline high-risk cytogenetic abnormalities (HRCA) if they maintained sequential cMRD negativity. In multivariate analysis, the absence of HRCA, complete response before ASCT, cMRDPRE-, and sustained cMRD negativity at both time points were independent predictors of longer TTNT.
Conclusions: Pre-ASCT cMRD assessment using both PET-CT and bone marrow FCM provides prognostic value in NDMM. This approach is particularly relevant in real-world settings where patients often receive prolonged induction therapy before ASCT.
Introduction
Over the past decade, substantial advancements have significantly improved survival rates for patients with newly diagnosed multiple myeloma (NDMM)1. These gains are attributed to enhanced diagnostic methods, the integration of novel therapeutic strategies, and the adoption of more sensitive techniques to monitor disease relapse. In this context, achieving bone marrow measurable residual disease (BM-MRD) negativity―assessed via flow cytometry (FCM) or molecular techniques―has become a critical prognostic indicator for long-term outcomes2. Autologous stem cell transplant (ASCT) continues to be an effective strategy that improves outcomes in patients with NDMM3–6. Evidence shows that the depth of response prior to ASCT influences both survival and relapse patterns7,8. However, while numerous studies have focused on MRD assessments after transplantation, data regarding the impact of pre-transplant MRD status on outcomes remains limited9–11. Additionally, relying solely on bone marrow assessments may underestimate the disease burden due to the spatial heterogeneity of multiple myeloma, which often includes focal lesions outside the marrow2,12. Functional imaging techniques, such as positron emission tomography-computed tomography (PET-CT) and MRI, provide valuable supplementary insights into MRD status, and their integration is now recommended by the International Myeloma Working Group (IMWG)13.
Most studies on bone marrow MRD have been conducted in Western settings, where stem cell mobilization typically occurs after four cycles of induction therapy, and stem cells are cryopreserved. In these cases, ASTC is performed either immediately or delayed, based on patient preference. In low- and middle-income countries (LMICs), however, stem cells are generally not cryopreserved. Due to out-of-pocket expenses, planned transplants are often delayed, and induction therapy is continued for longer periods. The relevance of composite MRD (cMRD) assessment in the context of extended induction therapy is not well understood. This study, therefore, aimed to assess the prognostic value of a composite response―integrating bone marrow MRD and PET-CT―conducted just before autologous ASCT and again at D+100 post-transplant in NDMM patients in a real-world LMIC setting.
Materials and Methods
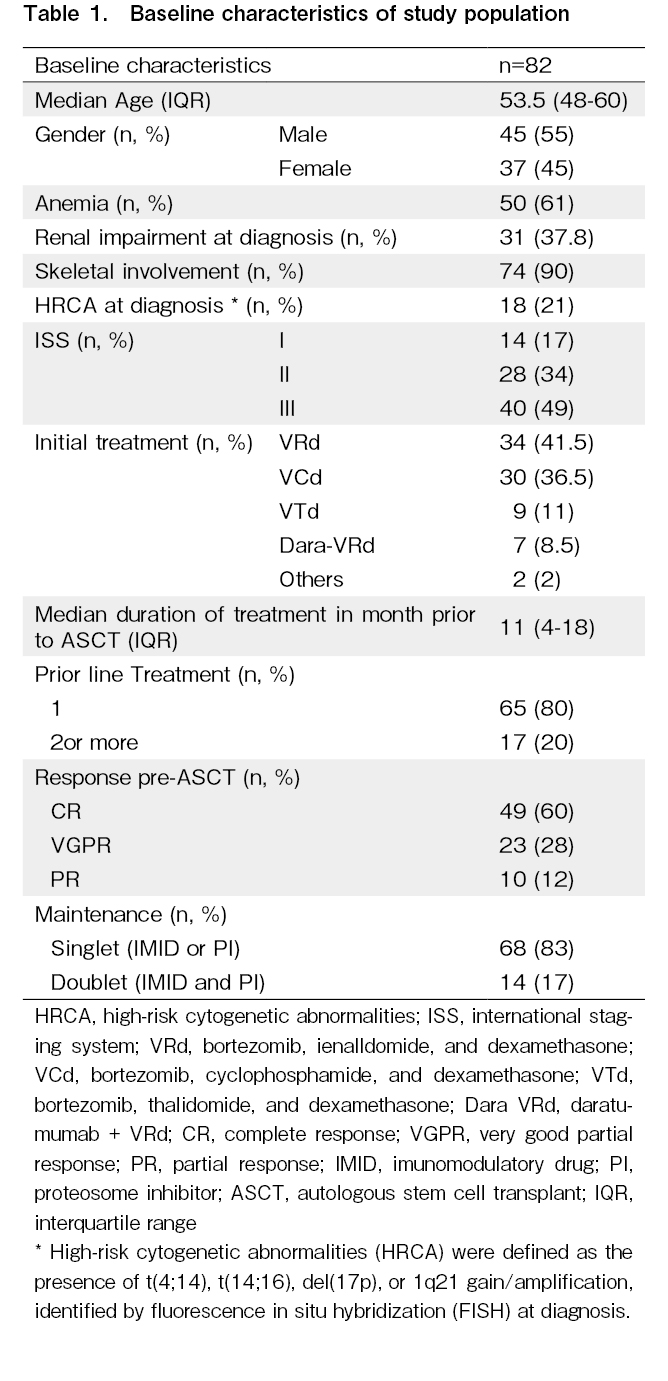
This retrospective cohort study included adult patients (age > 18 years) with newly diagnosed multiple myeloma who underwent both PET-CT imaging and BM-MRD assessment immediately before ASCT at our centre from January 2017 to June 2023. The study was approved by the Institutional Ethics Committee, PGIMER, Chandigarh vide letter no. IEC-12/2020-1868. Informed consent was taken from each patient at the time of initiation of treatment regarding the use of their outcome data for publication. Patients achieving less than a Partial Response before ASCT, based on IMWG criteria, were excluded.
High-risk cytogenetic abnormalities (HRCA) were defined as the presence of t(4;14), t(14;16), del(17p), or 1q21 gain/amplification, identified by fluorescence in situ hybridization (FISH) at diagnosis. BM-MRD and PET-CT imaging were performed immediately before ASCT and again 100 days post-transplant.
BM-MRD detection utilised a two-tube FCM assay with a 10-color antibody panel (CD38,
Baseline characteristics were summarised descriptively, with continuous variables as medians and interquartile ranges (IQRs) and categorical variables as percentages. Time to next treatment (TTNT) was defined as the interval from ASCT to initiation of new therapy due to disease progression, excluding maintenance changes due to side effects. Next treatment was initiated at the time of clinical relapse or significant paraprotein relapse based on IMWG criteria16. Overall survival was measured from diagnosis to death. Survival probabilities were estimated using Kaplan-Meier analysis, with group differences evaluated via log-rank tests. Cox proportional hazards regression models were applied for univariate and multivariate analyses to identify predictors, with a 5% significance level. Statistical analyses were conducted using SPSS version 24. The outcomes of patients who achieved both BM-MRD and PET negativity before ASCT i.e. composite MRD-negative (cMRDPRE-), or who had positive results on either of the modalities, i.e. (cMRDPRE+), those who achieved both BM-MRD and PET negativity at day 100 ASCT i.e. cMRDPOST- and those remaining positive on either/both modality i.e. cMRDPOST+ were analysed and compared.
Results
Baseline characteristics of the study population
A total of 106 patients with NDMM underwent ASCT during the study period. Five patients with progressive disease, six who lacked pre-transplant cMRD assessment and 13 who lacked post-transplant cMRD assessments were excluded, and 82 patients were included in the analysis (
Bone marrow measurable residual disease and PET-CT response before and after ASCT
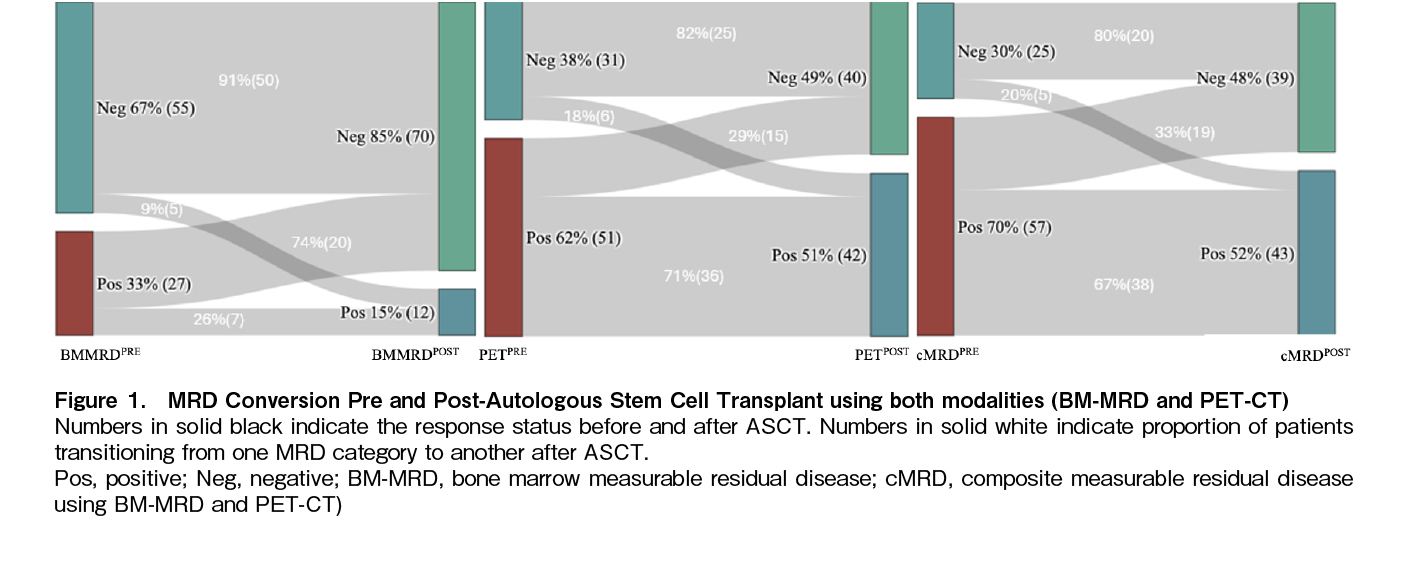
In the study cohort of 82 patients, 55 (67%) were negative for bone marrow measurable residual disease (BM-MRDPRE-) before ASCT, while 31 (38%) were negative on PET-CT (PETPRE-). A comparison of baseline disease characteristics between the cMRDPRE- versus cMRDPRE+ and cMRDPOST- versus cMRDPOST+ cohorts did not reveal any significant differences with respect to age, International Staging System, HRCA or renal involvement or duration of anti-myeloma therapy (
Prognostic impact of composite MRD assessment at pre-ASCT and post-ASCT time points
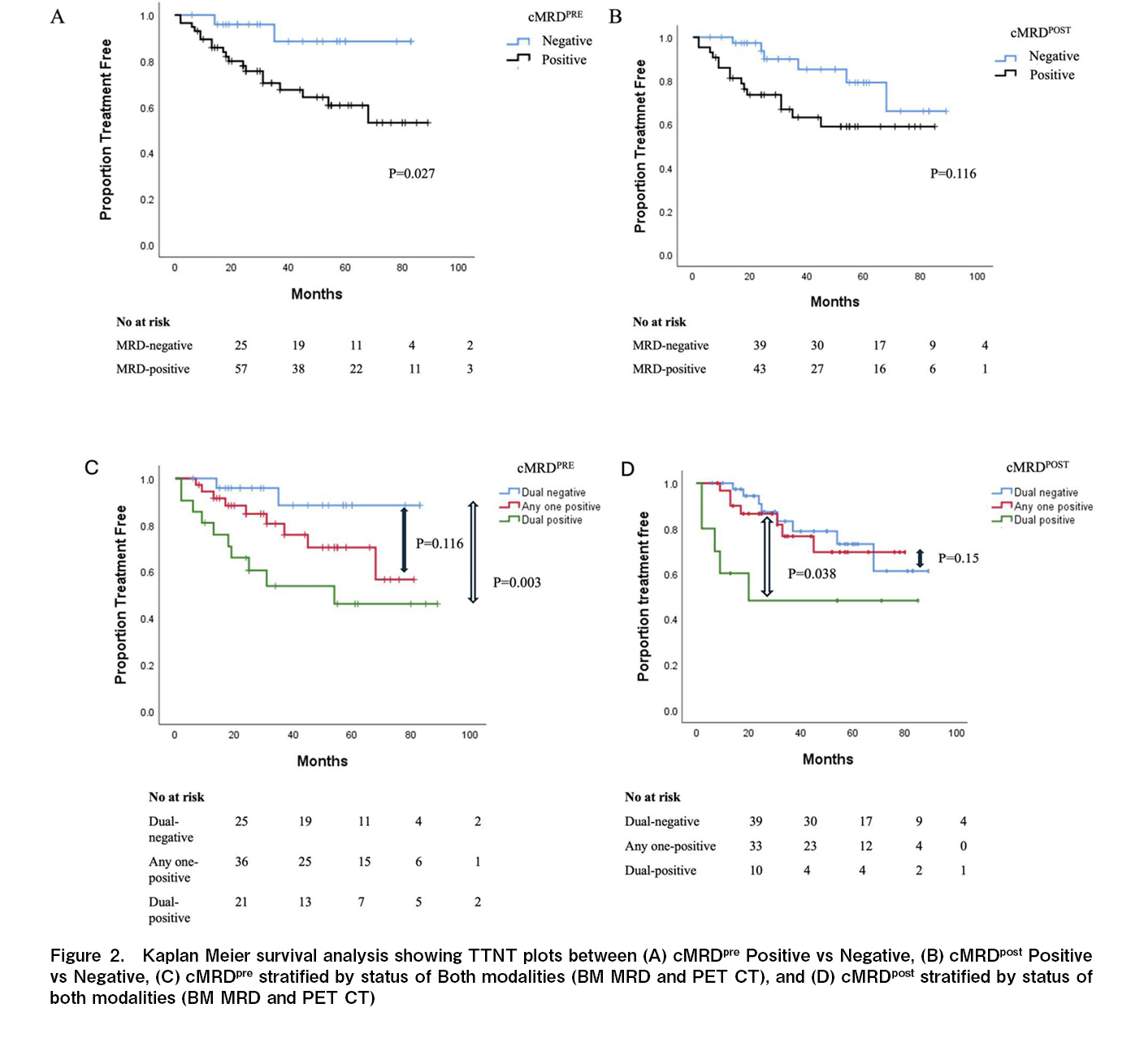
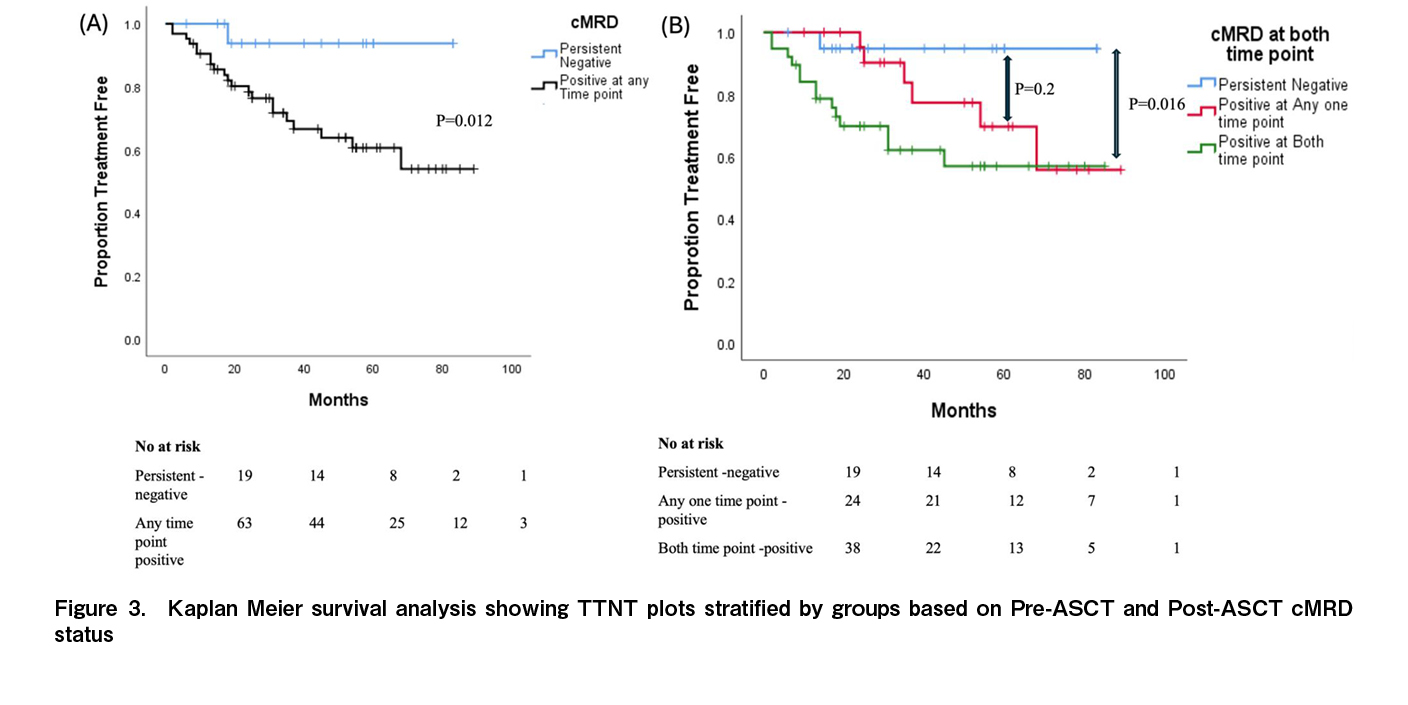
TTNT and overall survival were not reached for the entire study population. At three years, the TTNT was significantly longer for the cMRDPRE- cohort compared to the cMRDPRE+ cohort [91% (C.I. 77-100) versus 67% (C.I. 52-80); p=0.027] while statistically significant difference was not observed at the post-ASCT time point [82% (C.I. 68-96) versus 65% (C.I. 51-69); p=0.116] (Figure 2(A)-(D),
Among patients with HRCA at diagnosis, the proportion achieving cMRDPRE- was 27.8% (5 out of 18) before ASCT, which increased to 44.5% (8 out of 18) after ASCT. The three-year TTNT was comparable between patients with and without baseline HRCA who attained cMRD-negative status at both time points. However, for patients who remained cMRD-positive at either time point, the presence of HRCA continued to negatively impact TTNT (
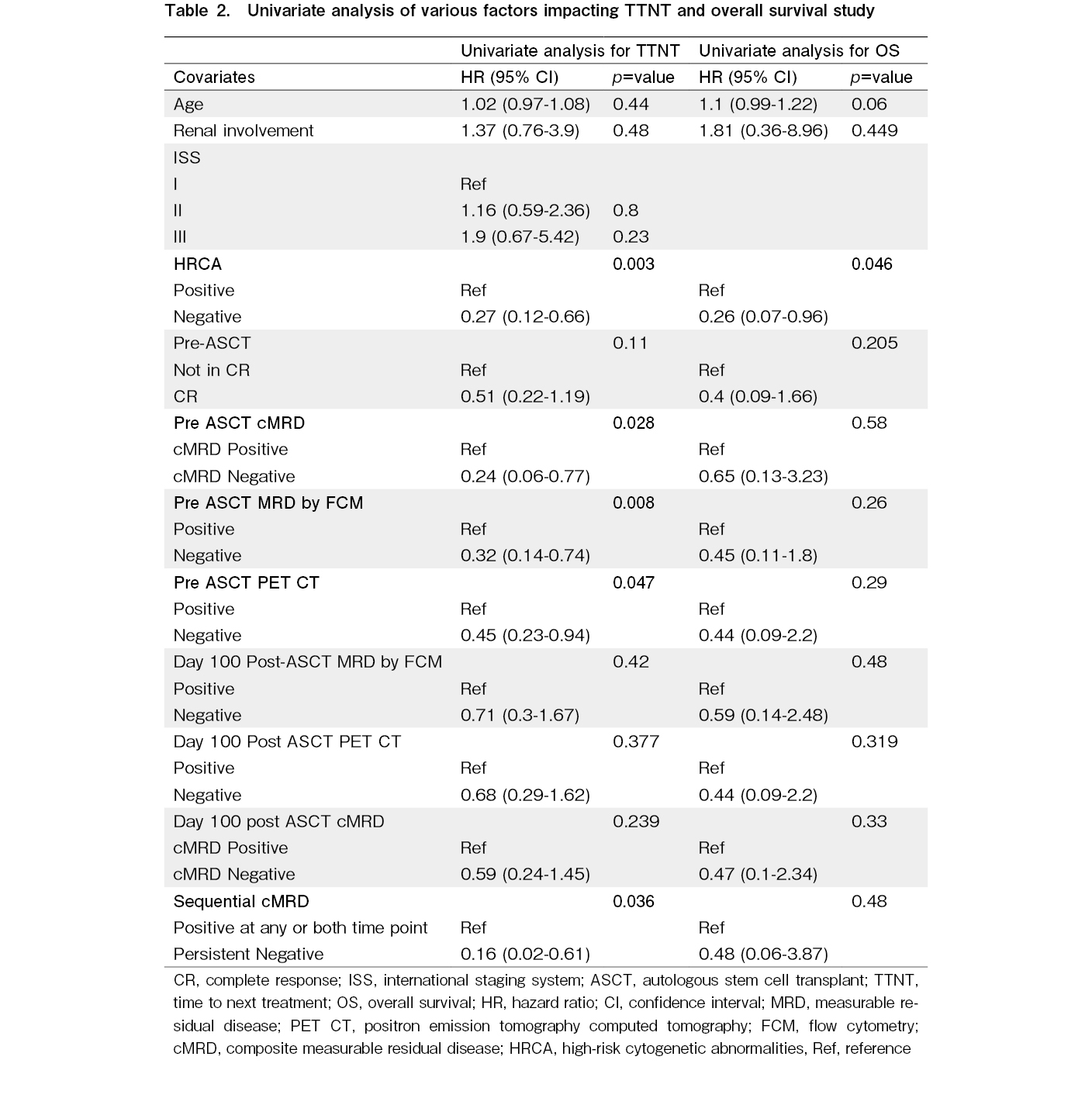
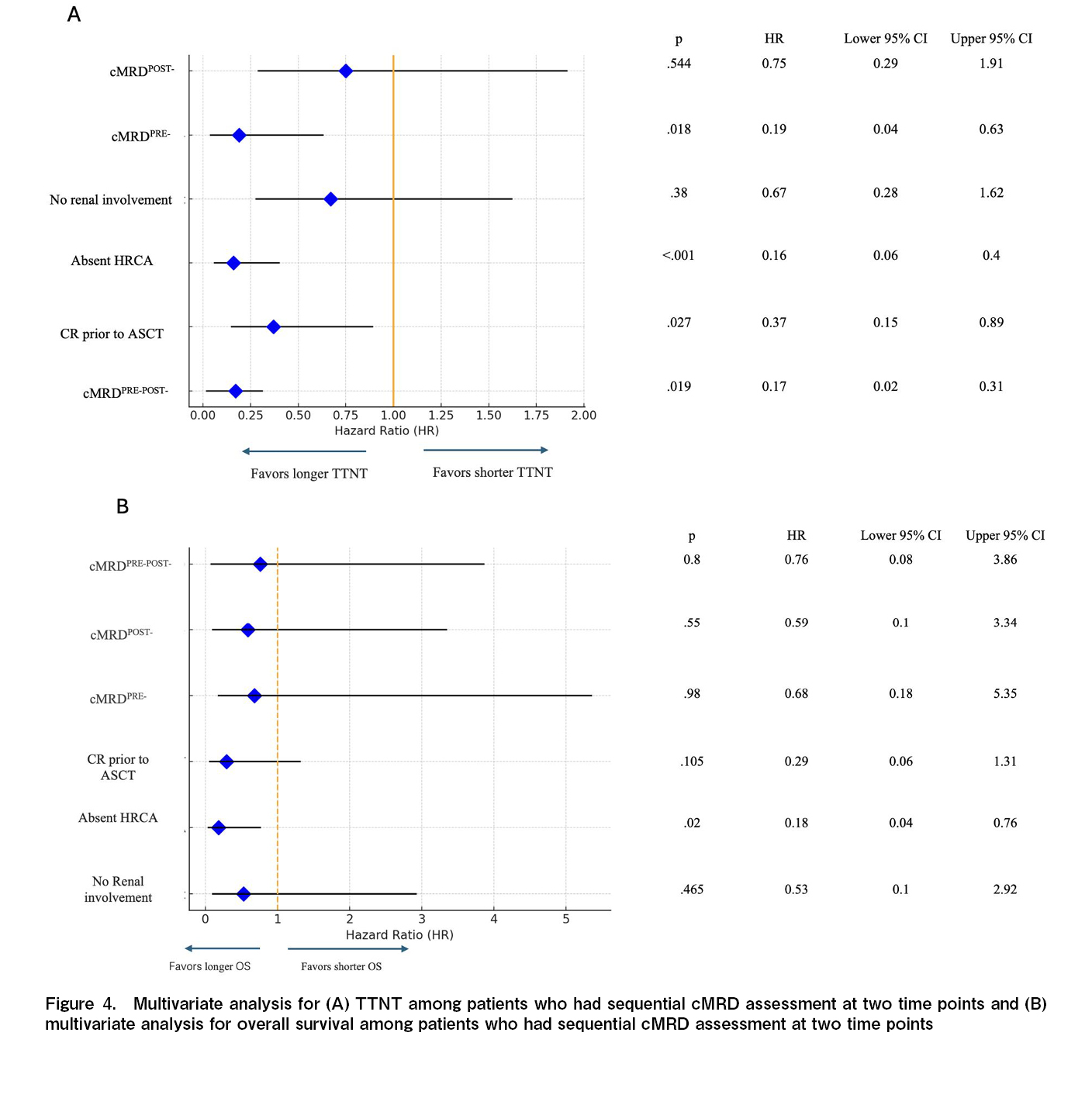
On the Cox univariate analysis, the absence of HRCA at diagnosis, pre-ASCT BM-MRD negativity, pre-ASCT PET negativity, cMRD-negative status before ASCT (cMRDPRE-), and cMRD negativity at both time points (cMRDPRE-cMRDPOST-) were identified as predictors of a longer TTNT: while the duration of antimyeloma therapy pre-ASCT and the choice of maintenance therapy post-ASCT did not impact TTNT (Table 2). In the multivariate analysis, the absence of HRCA at diagnosis was associated longer TTNT [Hazard Ratio (HR) of 0.16 (Confidence interval (CI) 0.06-0.41; p<0.001)]. Additionally, achieving CR before ASCT had an HR of 0.37 (CI 0.15-0.89; p=0.027), cMRD negativity before ASCT had an HR of 0.19 (CI 0.04-0.63; p=0.018), and sustained MRD negativity at both time point (cMRDPRE-cMRDPOST-) had HR of 0.17 (CI 0.02-0.31; p=0.019), indicating that these were independent predictors of longer TTNT (Figure 4).
In contrast, cMRDPOST- was not a significant predictor of longer TTNT in the multivariate analysis (Figure 4(A)). Among all the factors analysed, only the presence of HRCA was significantly associated with overall survival in the multivariate analysis (Figure 4(B)).
Discussion
MRD has become a critical surrogate marker for progression-free and overall survival in patients with multiple myeloma, serving as an essential endpoint in treatment17,18. However, the differential prognostic impact of various time points of achieving MRD negativity in the bone marrow and imaging negativity remains unclear. Most prior studies have relied on bone marrow MRD assessments conducted three months post-ASCT for prognostic predictions10,19. Our findings indicate that among patients with newly diagnosed multiple myeloma (NDMM) undergoing ASCT, TTNT varied based on when cMRD negativity was achieved. Specifically, those who maintained sequential cMRD negativity at both the time points had the most favourable prognosis. Additionally, cMRD negativity before ASCT was predictive of longer TTNT, with both BM-MRDPRE and pre ASCT PET negativity independently predictive of longer TTNT on univariate analysis. These results suggest that assessing cMRD negativity prior to ASCT may help identify a subgroup of patients who will have a longer time to next treatment following ASCT consolidation with standard immunomodulatory (IMID) drug and/or proteasome inhibitor (PI) maintenance in the setting where prolonged inductions are given prior to ASCT consolidation.
Unlike patients with most lymphomas where the time points for interim and follow-up image based response assessments are well validated, the timing and frequency of BM-MRD and imaging for response assessment in multiple myeloma remains uncertain. Most data suggest that sequential evaluations are more informative from a prognostic stand point20. IMWG defines sustained MRD negativity as consecutive negative MRD result in bone marrow, confirmed ≥ 1 year apart13. Sequential MRD studies using bone marrow and PET-CT before and after ASCT have a potential to analyse the efficacy of ASCT in the eradication of residual disease. In the current study patients who attain an early cMRD negativity had the longest time to next treatment, cMRDPRE was a stronger predictor of TTNT as compared to cMRDPOST-. Contrary to these findings the PRIMeR study reported, that patients achieving BM-MRDneg by multiparametric flow cytometry (MFC) at 1-year post-ASCT had the best outcomes rather than pre-maintenance and pre-ASCT time points. In the PRIMeR cohort, 42% of patients attained BM-MRD negativity at pre ASCT time point compared to 67% BM-MRD negativity in the current cohort. The long median duration of induction (~11 months in the current study compared to ~6 months in the PRIMeR cohort) may have led to a higher proportion of patients who were already MRD-negative at the pre-ASCT time point21. This extended induction period is particularly common in LMIC settings, where patients generally lack health insurance, and treatment costs are out-of-pocket, often resulting in delayed autologous transplants and the pre-ASCT time point may be particularly significant for prognostication in such settings. While the additional costs of BM-MRD and PET-CT imaging are significant, this needs to be viewed against the costs of subsequent lines of treatment. Majority of patients who relapse after IMID and Proteasome inhibitor exposure have limited treatment options in the real world. Given the ability of sequential cMRD to identify a subgroup of patients who have dismal outcome (Figure 3), a careful consideration needs to be given on the choice of maintenance strategies (single versus doublet maintenance) as well as the frequency of follow-up investigations and survelliance startegies for disease monitoring in patients who remain cMRD positive. However the utility of these strategies based on cMRD results need validation in prospective studies.
It is well known that while BM-MRD provides disease assessment at single cell resolution level at a medullary level, imaging modalities like PET-CT are a reflection of a global marrow activity and also extramedullary disease status. Therefore the two modalities have a potential of being discordant and the same was observed in the current and various other studies that have simultaneously analysed BM-MRD and PET-CT22. In the IMAJEM study that reported outcomes based on imaging response in patients receiving VRd based induction, the discordance between the BM-MRD and PET modalities at the pre-ASCT time point was 39.5%, compared to 44% in our study23. Similar high discordance rates between BM-MRD and PET were also reported in trials that utilised quadruplet combinations24. However, in other studies that used next-generation sequencing with a cut off 1 in 106 clonal plasma cells to define BM-MRD negativity, this discordance reduced to ~10%25. These finding suggests the complementary utility of PET for response assessment in the real world setting until more sensitive methods of BM-MRD assessment become widely available.
The current study also reported the comparative impact of ASCT on BM-MRD versus PET conversion rates in a real world patient population predominantly treated with a Proteosome Inhibitor –
The presence of HRCAs remained a significant factor for overall survival in the current study, as previously reported33,34. However, the TTNT and OS for patients who maintained sequential cMRD negativity at both assessment points were comparable amongst patients with and without HRCAs in the current study. This suggests that achieving cMRD negativity in both bone marrow MFC and PET-CT may help offset the adverse prognostic effect of HRCAs. A cMRD-guided approach before ASCT, including intensified treatment for MRD-positive patients with HRCAs, could improve outcomes and is currently being tested in several ongoing trials35,36.
The findings of this study should be interpreted considering the inherent limitations of a retrospective analysis with a small sample size, as well as the strengths of the real-world setting in which the data were collected outside of a clinical trial. Given the retrospective nature of the study with lack of consistent follow up intervals, the current study utilised TTNT rather than Progression-Free Survival for the comparison of outcomes. Although MRD detection sensitivity was 1 in 100,000 cells, baseline PET status was not included in the analysis, These limitations reflect scenarios commonly encountered in most real-world settings. Additionally, only a limited number of patients received the more effective quadruplet regimens, now considered the standard of care for frontline treatment of NDMM in high-income countries. This limitation, however, underscores the relevance of studying outcomes in settings where resource constraints necessitate the use of triplet regimens. Furthermore, most patients underwent ASCT later than usual, after receiving a higher median number of induction cycles. While this might have influenced pre-ASCT and subsequent response assessments, it also provides a unique opportunity to evaluate the prognostic impact of bone marrow and imaging MRD in the context of extended induction therapy – a scenario more commonly encountered in LMICs due to delayed access to transplants. By addressing the gaps in composite response assessments at multiple time points, future prospective research can better guide treatment strategies in resource-limited settings, enhancing global equity in myeloma care.
In summary, our findings highlight the importance of PET-CT and BM-MRD in assessing residual disease in NDMM patients. Additionally, the pre-ASCT time point may be particularly crucial for sequential MRD evaluation and future prognostication, especially in settings where longer initial treatments are administered before ASCT.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
DL is one of the editors of Blood Cell Therapy. He was not involved in the editorial evaluation or decision to accept this article for publication.
Acknowledgments
Grammarly software was used for English editing.
Author Contribution
RNS and AJ analyzed the data and wrote the initial draft of the manuscript. All authors were involved in patient recruitment, reviewing, and approving the final manuscript. RNS and AJ have equally contributed to the manuscript.
Acknowledgments
Grammarly software was used for English editing.
Data availability statement
Data will be made available on reasonable request by contacting the corresponding author.
References
1.Puertas B, González-Calle V, Sobejano-Fuertes E, Escalante F, Queizán JA, Bárez A, et al. Novel Agents as Main Drivers for Continued Improvement in Survival in Multiple Myeloma. Cancers (Basel). 2023; 15: 1558.
2.Rasche L, Alapat D, Kumar M, Gershner G, McDonald J, Wardell CP, et al. Combination of flow cytometry and functional imaging for monitoring of residual disease in myeloma. Leukemia. 2019; 33: 1713-22.
3.Malhotra P, Yanamandra U, Khadwal A, Prakash G, Lad D, Law AD, et al. Autologous Stem Cell Transplantation for Multiple Myeloma: Single Centre Experience from North India. Indian J Hematol Blood Transfus. 2018; 34: 261-7.
4.Asrar MM, Lad DP, Bansal D, Prinja S, Khadwal A, Prakash G, et al. Health-related quality of life in transplant eligible multiple myeloma patients with or without early ASCT in the real-world setting. Leuk Lymphoma. 2021; 62: 3271-7.
5.Prinja S, Kaur G, Malhotra P, Jyani G, Ramachandran R, Bahuguna P, et al. Cost-Effectiveness of Autologous Stem Cell Treatment as Compared to Conventional Chemotherapy for Treatment of Multiple Myeloma in India. Indian J Hematol Blood Transfus. 2017; 33: 31-40.
6.Dhakal B, Shah N, Kansagra A, Kumar A, Lonial S, Garfall A, et al. ASTCT Clinical Practice Recommendations for Transplantation and Cellular Therapies in Multiple Myeloma. Transplant Cell Ther. 2022; 28: 284-93.
7.Kaddoura M, Dingli D, Buadi FK, Lacy MQ, Gertz MA, Dispenzieri A, et al. Prognostic impact of posttransplant FDG PET/CT scan in multiple myeloma. Blood Adv. 2021; 5: 2753-9.
8.Liu J, Yan W, Fan H, Xu J, Li L, Du C, et al. Clinical Benefit of Autologous Stem Cell Transplantation for Patients with Multiple Myeloma Achieving Undetectable Minimal Residual Disease after Induction Treatment. Cancer Res Commun. 2023; 3: 1770-80.
9.Paiva B, Manrique I, Dimopoulos MA, Gay F, Min CK, Zweegman S, et al. MRD dynamics during maintenance for improved prognostication of 1280 patients with myeloma in the TOURMALINE-MM3 and -MM4 trials. Blood. 2023; 141: 579-91.
10.de Tute RM, Pawlyn C, Cairns DA, Davies FE, Menzies T, Rawstron A, et al. Minimal Residual Disease After Autologous Stem-Cell Transplant for Patients With Myeloma: Prognostic Significance and the Impact of Lenalidomide Maintenance and Molecular Risk. J Clin Oncol. 2022; 40: 2889-900.
11.Oliva S, Bruinink DHO, Rihova L, D'Agostino M, Pantani L, Capra A, et al. Minimal residual disease assessment by multiparameter flow cytometry in transplant-eligible myeloma in the EMN02/HOVON 95 MM trial. Blood Cancer J. 2021; 11: 106.
12.Rasche L, Chavan SS, Stephens OW, Patel PH, Tytarenko R, Ashby C, et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat Commun. 2017; 8: 268.
13.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016; 17: e328-46.
14.Lad DP, Malhotra P, Patil AN, Nampoothiri RV, Kasudhan KS, Khadwal A, et al. Long-term outcomes of innovator versus generic melphalan formulation in autologous hematopoietic cell transplantation for multiple myeloma. Hematol Oncol Stem Cell Ther. 2021; 14: 114-8.
15.Thakral B, Saluja K, Sharma RR, Malhotra P, Varma N, Marwaha N, et al. Extended Storage of Liquid-Preserved Stem Cells at 4°C Results in Good Engraftment in Patients of Multiple Myeloma Undergoing Autologous Stem Cell Transplantation. Laboratory Medicine. 2005; 36: 650-1.
16.Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011; 117: 4691-5.
17.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016; 149: 315-52.
18.Jain A, Jain A, Malhotra P. Re-defining Prognosis of Hematological Malignancies by Dynamic Response Assessment Methods: Lessons Learnt in Chronic Myeloid Leukemia, Hodgkin Lymphoma, Diffuse Large B Cell Lymphoma and Multiple Myeloma. Indian J Hematol Blood Transfus. 2020; 36: 447-57.
19.Nahi H, Afram G, Uttervall K, Lockmer S, Tätting L, Gahrton G, et al. Minimal residual disease status is the prognostic determinant following high-dose treatment for patients with multiple myeloma. Cancer Med. 2023; 12: 20736-44.
20.Szalat R, Anderson K, Munshi N. Role of minimal residual disease assessment in multiple myeloma. Haematologica. 2024; 109: 2049-59.
21.Pasquini MC, Wallace PK, Logan B, Kaur M, Tario JD, Howard A, et al. Minimal Residual Disease Status in Multiple Myeloma 1 Year After Autologous Hematopoietic Cell Transplantation and Lenalidomide Maintenance Are Associated With Long-Term Overall Survival. J Clin Oncol. 2024; 42: 2757-68.
22.Meseha M, Hoffman J, Kazandjian D, Landgren O, Diamond B. Minimal Residual Disease-Adapted Therapy in Multiple Myeloma: Current Evidence and Opinions. Curr Oncol Rep. 2024; 26: 679-90.
23.Moreau P, Attal M, Caillot D, Macro M, Karlin L, Garderet L, et al. Prospective Evaluation of Magnetic Resonance Imaging and [(18)F]Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography at Diagnosis and Before Maintenance Therapy in Symptomatic Patients With Multiple Myeloma Included in the IFM/DFCI 2009 Trial: Results of the IMAJEM Study. J Clin Oncol. 2017; 35: 2911-8.
24.Moreau P, Zweegman S, Perrot A, Hulin C, Caillot D, Facon T, et al. Evaluation of the Prognostic Value of Positron Emission Tomography-Computed Tomography (PET-CT) at Diagnosis and Follow-up in Transplant-Eligible Newly Diagnosed Multiple Myeloma (TE NDMM) Patients Treated in the Phase 3 Cassiopeia Study: Results of the Cassiopet Companion Study. Blood. 2019; 134 (Suppl 1): 692.
25.Fonseca R, Arribas M, Wiedmeier-Nutor JE, Kusne YN, González Vélez M, Kosiorek HE, et al. Integrated analysis of next generation sequencing minimal residual disease (MRD) and PET scan in transplant eligible myeloma patients. Blood Cancer J. 2023; 13: 32.
26.Zamagni E, Nanni C, Dozza L, Carlier T, Bailly C, Tacchetti P, et al. Standardization of (18)F-FDG-PET/CT According to Deauville Criteria for Metabolic Complete Response Definition in Newly Diagnosed Multiple Myeloma. J Clin Oncol. 2021; 39: 116-25.
27.Nørgaard JN, Abildgaard N, Lysén A, Tsykunova G, Vangsted AJ, João C, et al. Intensifying treatment in PET-positive multiple myeloma patients after upfront autologous stem cell transplantation. Leukemia. 2023; 37: 2107-14.
28.Yanamandra U, Reddy Gorla AK, Agrawal K, Mittal BR, Prakash G, Khadwal AR, et al. Prognostic significance of extramedullary disease (EMD) detected on pre-transplant 18F-FDG PET/CT in patients with multiple myeloma: Results of PIPET-M trial. Med J Armed Forces India. 2023; 79: 672-8.
29.Kraeber-Bodéré F, Zweegman S, Perrot A, Hulin C, Caillot D, Facon T, et al. Prognostic value of positron emission tomography/computed tomography in transplant-eligible newly diagnosed multiple myeloma patients from CASSIOPEIA: the CASSIOPET study. Haematologica. 2023; 108: 621-6.
30.Zamagni E, Patriarca F, Nanni C, Zannetti B, Englaro E, Pezzi A, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011; 118: 5989-95.
31.Zamagni E, Nanni C, Mancuso K, Tacchetti P, Pezzi A, Pantani L, et al. PET/CT Improves the Definition of Complete Response and Allows to Detect Otherwise Unidentifiable Skeletal Progression in Multiple Myeloma. Clin Cancer Res. 2015; 21: 4384-90.
32.Sachpekidis C, Hillengass J, Goldschmidt H, Wagner B, Haberkorn U, Kopka K, et al. Treatment response evaluation with (18)F-FDG PET/CT and (18)F-NaF PET/CT in multiple myeloma patients undergoing high-dose chemotherapy and autologous stem cell transplantation. Eur J Nucl Med Mol Imaging. 2017; 44: 50-62.
33.Smallbone P, Clugston S, De Kraa R, Purtill D, Wright M, Leahy MF, et al. Presence of Multiple High Risk Cytogenetic Abnormalities Is Associated with Rapid Progression and Shorter Survival in Newly Diagnosed Multiple Myeloma. Blood. 2020; 136 (Suppl 1): 23.
34.Singh C, Panakkal V, Sreedharanunni S, Jandial A, Jain A, Lad D, et al. Presentation and Impact of Double and Triple hit Cytogenetics in Patients With Multiple Myeloma in the Real World. Clin Lymphoma Myeloma Leuk. 2022; 22: e685-90.
35.Derman BA, Major A, Cooperrider J, Jiang K, Ramsland A, Karrison T, et al. Discontinuation of maintenance therapy in multiple myeloma guided by multimodal measurable residual disease negativity (MRD2STOP). Blood Cancer J. 2024; 14: 170.
36.Meseha M, Hoffman J, Kazandjian D, Landgren O, Diamond B. Minimal Residual Disease-Adapted Therapy in Multiple Myeloma: Current Evidence and Opinions. Curr Oncol Rep. 2024; 26: 679-90.
Search
News