Volume 8 (2025) Issue 3 No.3 Pages 228-233
Abstract
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative treatment for hematological malignancies. Myeloablative conditioning (MAC) is generally preferred whenever possible to reduce the risk of relapse in acute leukemia1. While the organ function criteria, comorbidity index, and performance status cutoffs are similar across centers, the age cutoff to offer MAC varies, typically ranging from 55 to 652,3. Given the absence of validated tools for frailty/geriatric assessment in HCT, the evaluation of physiologic age remains subjective. The effects of race as both a genetic and socio-economic construct on HCT outcomes have yielded mixed results. The Centre for International Blood and Marrow Transplant Research (CIBMTR) studies have indicated that survival rates are worse for Black and Hispanic patients compared to White and Asian patients within the first year after HCT in the US. However, the survival rates eventually equalize for those who survive beyond one year4–6. Other studies have shown that race has no impact on HCT outcomes7,8. However, most of these studies have included adult patients regardless of age and were performed in resource-rich centers. Studies done in the South Asian population have acknowledged the challenges associated with MAC regimens and have shown worse overall survival and higher mortality with these regimens compared to reduced intensity conditioning (RIC)9,10. In most centers in India, the age cutoff for offering MAC is 45 years. The biological reasons for this are not entirely clear. The influence of socioeconomic factors and supportive care on outcome differences also requires further investigation. We hypothesize that the outcomes for South Asians aged 45 and older receiving a MAC regimen for hematological malignancies are worse compared to other populations, even when treated in a public funded universal healthcare system with centralized uniform treatment criteria.
Materials and Methods
This retrospective single-centre study was conducted at The Leukemia/Bone Marrow Transplant Program of British Columbia, Vancouver, Canada. We included all patients (age ≥ 45 years) undergoing myeloablative conditioning for hematological malignancies from January 2011 to December 2022. Institutional Research Ethics Board approval was obtained before the study. Myeloablative conditioning included total body irradiation (TBI) ≥ 8 Gy fractionated or Busulfan > 6.4 mg/kg IV11. Chemotherapy dosing was as per adjusted body weight 50 if the actual body weight was more than ideal. Baseline patient characteristics (age, sex, race, body mass index (BMI), hematopoietic cell transplantation-comorbidity index (HCT-CI), performance status, disease, and disease status before transplant), transplant details (donor details, cytomegalovirus (CMV) matching, blood group matching, date of transplant, conditioning regimen, graft versus host disease (GVHD) prophylaxis, and stem cell dose) and events (acute and chronic GVHD, death, and relapse) were recorded. GVHD prophylaxis included standard calcineurin inhibitor (CNI) + methotrexate-based prophylaxis for matched sibling donor (MSD) – and matched unrelated donor (MUD) – HCT. Rabbit Antithymocyte globulin (ATG) (ThymoglobulinⓇ; Sanofi-Aventis, Laval, Canada) was added for GVHD prophylaxis for MUD-HCT from 2016 and MSD-HCT from 2018 onwards. The GVHD prophylaxis for haploidentical HCT included post-transplant cyclophosphamide + CNI + mycophenolate. Race was self-reported by the patient and classified as per the CIBMTR definition and guidance on using standards for race-based data collection and health reporting in Canada12. The primary outcome was overall survival (OS). OS was defined as the time from the day of hematopoietic infusion to death from any cause or last documented follow-up. Secondary outcomes were non-relapse mortality (NRM), cumulative incidence of grade 2-4 acute GVHD (graded as per CIBMTR consensus criteria), moderate-severe chronic GVHD (graded as per National Institutes of Health consensus criteria) and relapse incidence (RI) with relapse as the competing risk. The baseline and the transplant characteristics were compared between cohorts using the Mann-Whitney, chi-square or Fisher's exact test. The survival analysis was done using Kaplan-Meier analysis and log-rank test. The GVHD, NRM, and RI rates were calculated using the cumulative incidence of competing events and the Gray test. A p-value of
Results
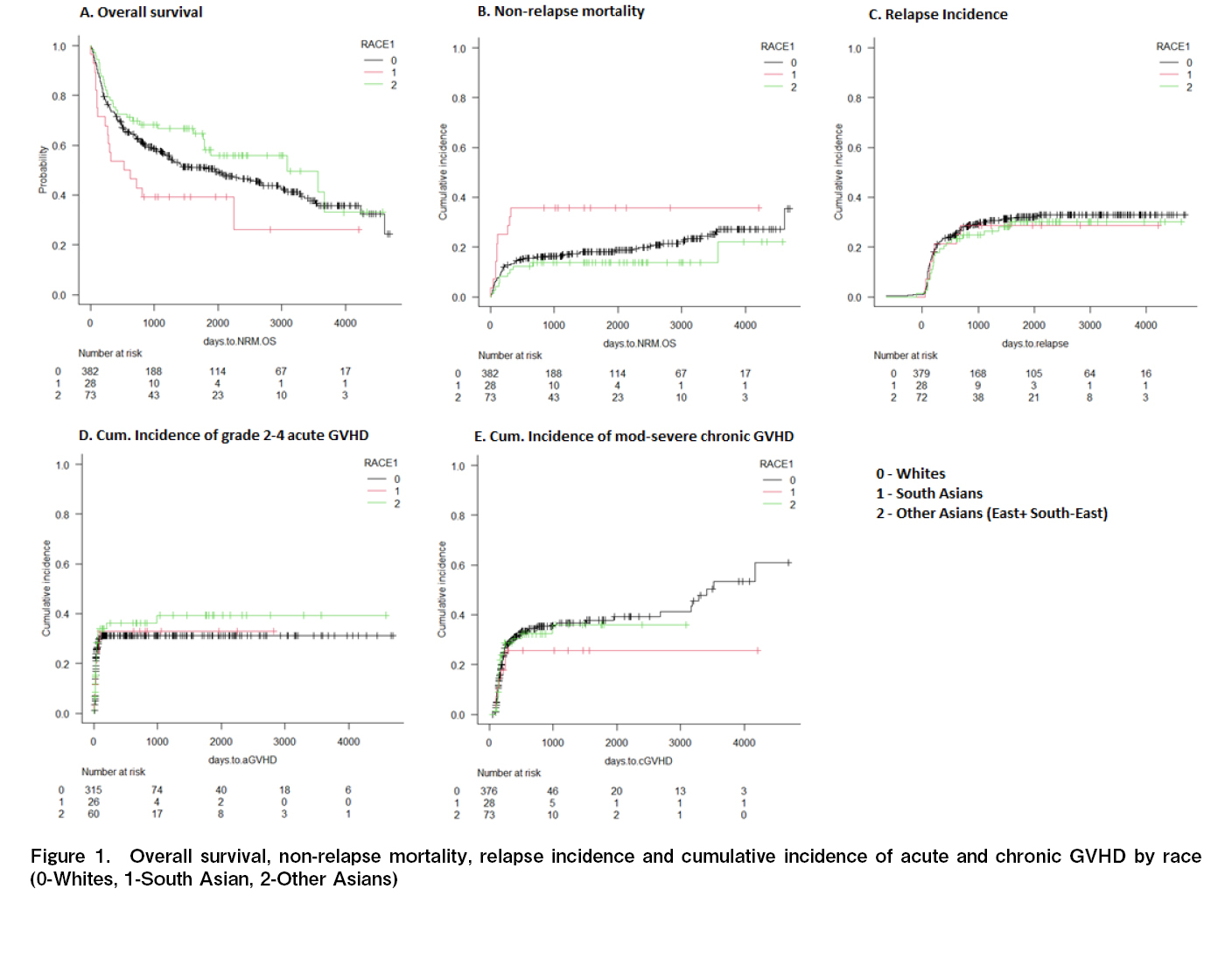
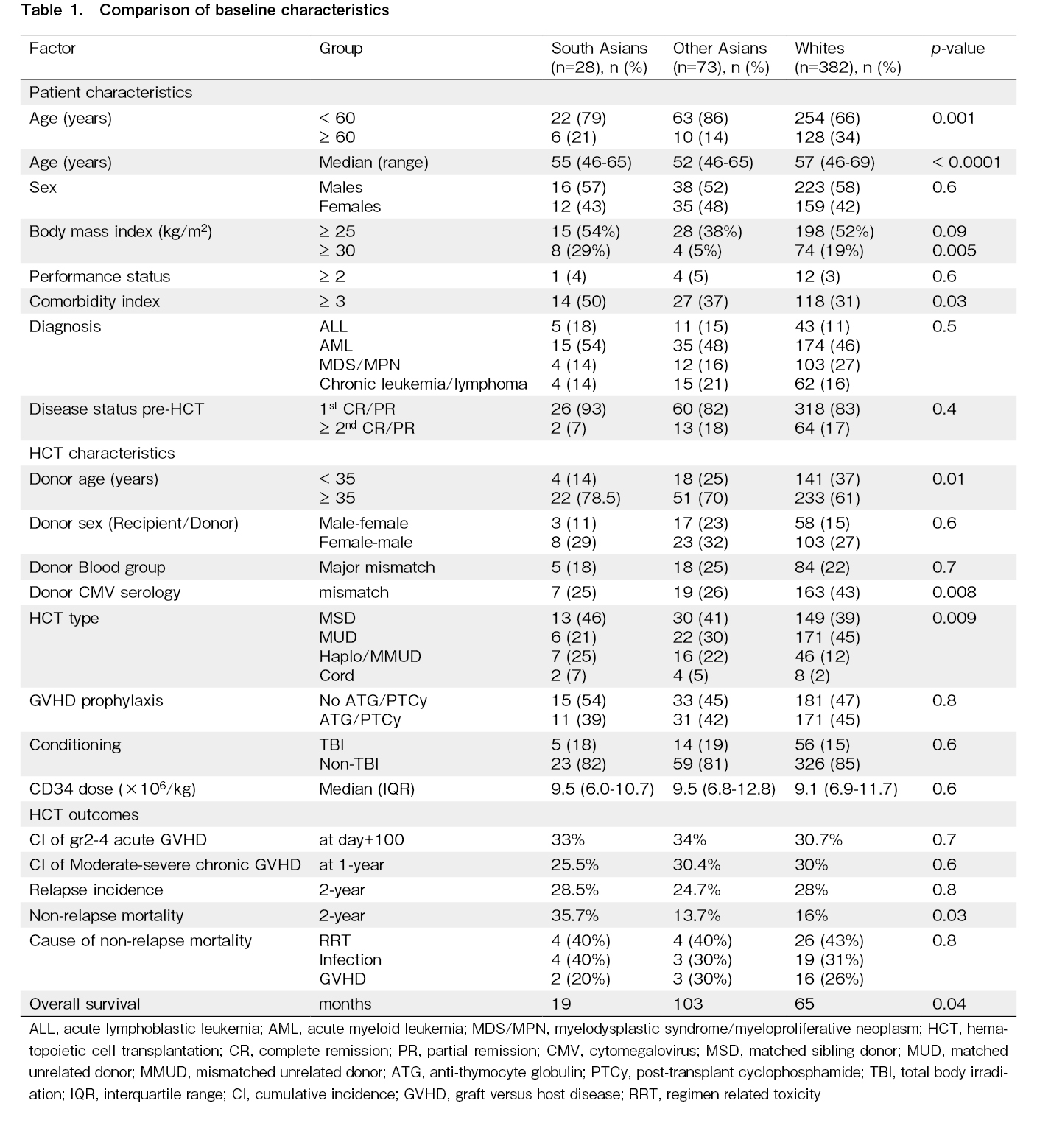
Of the 483 patients, there were 28 (5.8%) South Asians (SA), 73 (15.1%), East/Southeast Asians (EA/SEA), and 382 (79.1%) Whites (W). The median age of the whole cohort was 56 years (range 46-69 years). The most typical indication for HCT was acute myeloid leukemia (46%), followed by myelodysplastic syndrome/myeloproliferative neoplasm (25%). Most HCTs were done for disease in first remission (84%). Most transplants were MSD (40%) and MUD-HCT (41%). A comparison of baseline characteristics of the patients across the three racial subgroups is summarised in Table 1. There was a lower proportion of older recipients (≥ 60 years of age) among Asians than among Whites (SA 21%, EA/SEA 14%, W 34%, p=0.001). The proportion of older donors (≥ 30 years of age) was higher among Asians compared to Whites (SA 78.5%, EA/SEA 70%, W 61%, p=0.01). The three groups were comparable in terms of the proportion of recipient and donor sex, and performance status (Eastern Cooperative Oncology Group score ≥ 2). The proportion of patients with HCT-CI ≥ 3 was significantly higher in SA (SA 50%, EA/SEA 37%, W 31%, p=0.03). The proportion of patients with obesity (BMI ≥ 30 kg/m2) was significantly higher in SA (SA 29%, EA/SEA 5%, W 19%,
The median OS was significantly shorter in SA (SA 19, EA/SEA 103, W 65 months, p=0.04) (Figure 1A). The 2-year NRM was highest in SA (SA 35.7%, EA/SEA 13.7%, W 16%, p=0.03) (Figure 1B). There was no significant difference in the cause of death attributed to infections, regimen-related toxicity, and GVHD in the three groups. The 2-year RI was also not significantly different (SA 28.5%, EA/SEA 24.7%, W 28%, p=0.8) (Figure 1C). The cumulative incidence of grade 2-4 acute and moderate-severe chronic GVHD was
Discussion
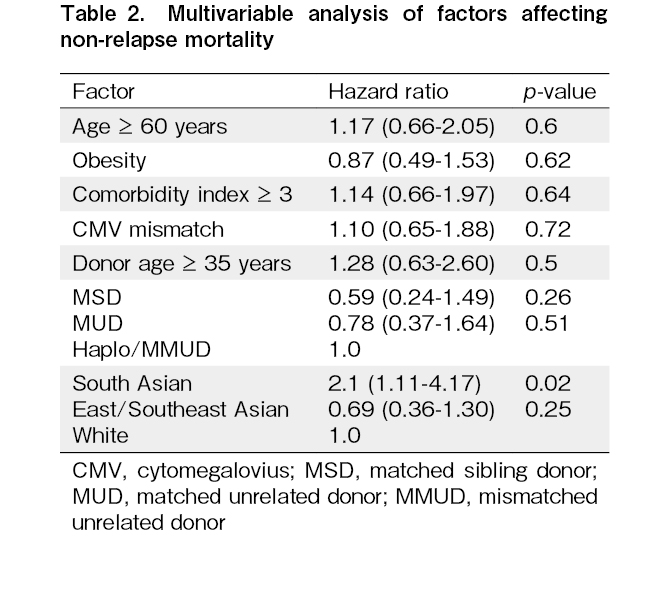
Our study shows that in patients older than 45 years, South Asians have higher mortality, and other Asians may have lower mortality after MAC-HCT. The high NRM is probably due to differences in comorbidities, with a higher proportion of South Asians having HCT-CI ≥ 3. South Asians were more likely to be obese as per the World Health Organization's definition. However, neither the comorbidity index nor obesity was identified as a risk factor affecting NRM on multivariable analysis. The high NRM with MAC regimens in South Asians has been demonstrated in studies from India, where RIC regimens are preferred in patients aged ≥ 45 years. In one study, the MAC regimen was associated with higher 100 day (18.4 vs 6%) and 1-year NRM (52.6 vs 20.9%) compared to reduced intensity chemotherapy (fludarabine/melphalan)10. British Columbia has a significant Asian population, with ~65.6% Whites, ~9.6% South Asians, and 18.6% East/Southeast Asians14. Its publicly funded healthcare system ensures equitable healthcare services and prescription medication access. The proportion of patients undergoing myeloablative conditioning was representative of the provincial population. Since we did not have data on the total number of patients eligible for HCT, it is difficult to determine if there was any racial disparity in the stem cell utilization rates. However, some centres in the US have reported selection bias in HCT and barriers to HCT in minority and low-income patients15. This could not have been the cause in our study, as the median income of Asians, including South Asians, was higher than the median national income16.
The impact of race as a determinant for outcomes following HCT has been a field of interest for decades. Early studies showed a higher mortality among the US Black population, driven by a higher relapse and GVHD17. This was thought to be related to unidentified immunological or socio-cultural parameters. Socio-economic factors were ruled out as a cause for inferior outcomes in Black patients in a subsequent study4. This study attributed the inferior outcomes in Black patients to pharmacogenomic or unmeasured comorbidities.
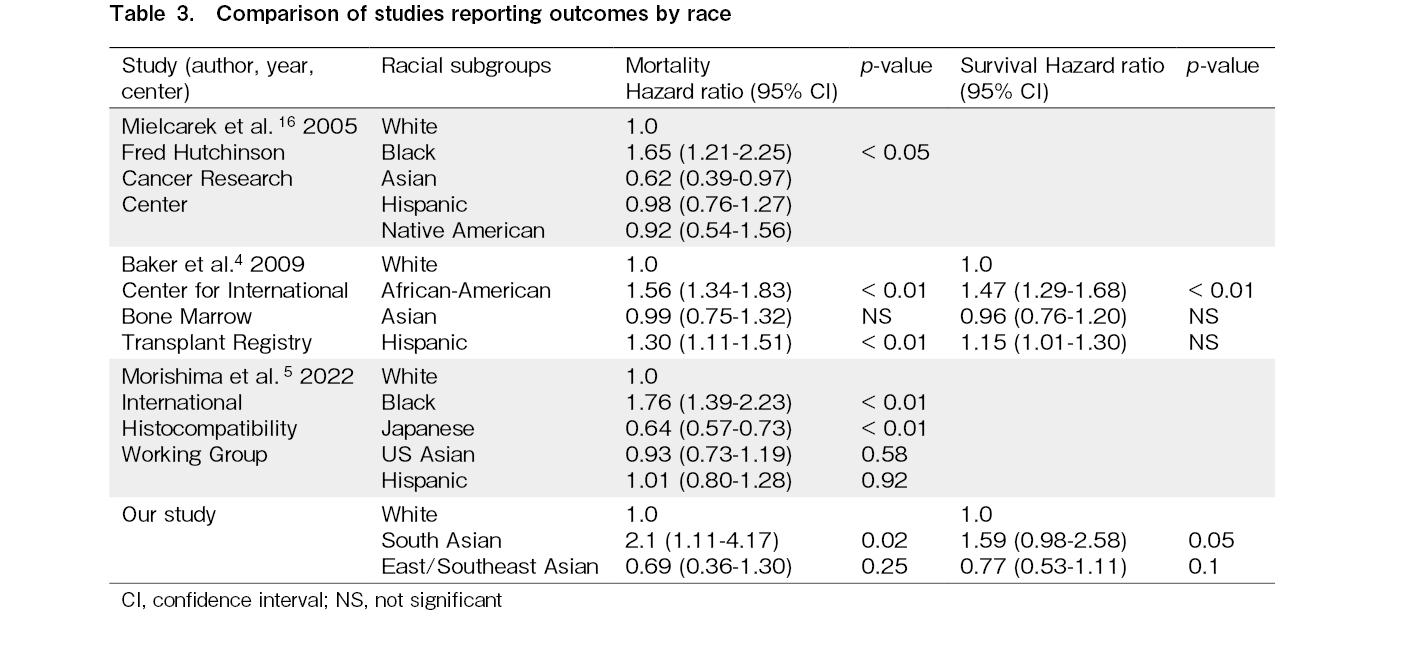
A recent study showed survival was superior for Japanese, intermediate for U.S. Asian, White, and Hispanic, and lowest for Black patients, with optimal matching for donor and human leukocyte antigen (HLA) characteristics5 (Table 3). The reasons for lower mortality in Japanese and US Asians and higher mortality in Blacks were attributed to the genetic conservation of HLA haplotypes in Japanese patients and donors compared to diverse haplotypes in other races. Another recent CIBMTR study showed no difference in outcomes by race or poverty in those surviving ≥ 1 year after HCT6. As our study shows, this could be attributed to higher mortality in the first year after HCT. These studies are limited by the underrepresentation of South Asians or the inclusion of patients of all age groups. Our study is limited by its retrospective nature and the relatively limited number of South Asian patients.
Our study confirms that South Asians aged ≥ 45 have worse survival after MAC-HCT. Supportive care in a publicly funded universal healthcare setting did not overcome the differences in the outcomes. Future studies should prospectively focus on the combined effect of comorbidities, frailty, and pharmacogenetics as a cause for this racial disparity in HCT outcomes.
Acknowledgments
Data coordinators Helena Nutakor and Nancy Roth
Author Contributions
SM, SN, YAM, TJN, and DL conceived and wrote the study; SM and DL analyzed the data and wrote the first draft of the manuscript. All authors contributed to patient care and manuscript writing.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
DL is one of the editors of Blood Cell Therapy. He was not involved in the editorial evaluation or decision to accept this article for publication.
References
1.Scott BL, Pasquini MC, Fei M, Fraser R, Wu J, Devine SM, et al. Myeloablative versus Reduced-Intensity Conditioning for Hematopoietic Cell Transplantation in Acute Myelogenous Leukemia and Myelodysplastic Syndromes-Long-Term Follow-Up of the BMT CTN 0901 Clinical Trial. Transplant Cell Ther. 2021; 27: 483.e1-6.
2.Hamadani M, Craig M, Awan FT, Devine SM. How we approach patient evaluation for hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010; 45: 1259-68.
3.Kanate AS, Perales MA, Hamadani M. Eligibility Criteria for Patients Undergoing Allogeneic Hematopoietic Cell Transplantation. J Natl Compr Canc Netw. 2020; 18: 635-43.
4.Baker KS, Davies SM, Majhail NS, Hassebroek A, Klein JP, Ballen KK, et al. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009; 15: 1543-54.
5.Morishima Y, Morishima S, Stevenson P, Kodera Y, Horowitz M, McKallor C, et al. Race and Survival in Unrelated Hematopoietic Cell Transplantation. Transplant Cell Ther. 2022; 28: 357.e1-6.
6.Blue BJ, Brazauskas R, Chen K, Patel J, Zeidan AM, Steinberg A, et al. Racial and Socioeconomic Disparities in Long-Term Outcomes in ≥1 Year Allogeneic Hematopoietic Cell Transplantation Survivors: A CIBMTR Analysis. Transplant Cell Ther. 2023; 29: 709.e1-11.
7.Herrity E, Singhabahu S, Remberger M, Novitzky-Basso I, Pasic I, Lam W, et al. Exploring Outcomes by Ethnicity in Allogeneic Hematopoietic Cell Transplantation. Transplant Cell Ther. 2024; 30: S325.
8.Sigmund AM, Zhao Q, Jiang J, Elder P, Benson DM, Rosko A, et al. Impact of Race and Geographic Area of Residence on Outcomes After Allogeneic Stem Cell Transplant. Front Oncol. 2022; 12: 801879.
9.Sharma SK, Choudhary D, Doval D, Khandelwal V, Patel A, Setia R, et al. Myeloablative Versus Reduced Intensity Conditioning Regimens for Allogeneic Hematopoietic Stem Cell Transplant for Acute Myeloid Leukemia and Myelodysplastic Syndrome: A Retrospective Analysis. Indian J Hematol Blood Transfus. 2021; 37: 472-8.
10.Ganapule A, Nemani S, Korula A, Lakshmi KM, Abraham A, Srivastava A, et al. Allogeneic Stem Cell Transplant for Acute Myeloid Leukemia: Evolution of an Effective Strategy in India. J Glob Oncol. 2017; 3: 773-81.
11.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009; 15: 1628-33.
12.CIBMTR. Forms Instruction Manual, Appendix I: Ethnicity and Race. 2015. https://www.manula.com/manuals/cibmtr/fim/1/en/topic/appendix-i-ethnicity-and-race [Accessed: 29 January 2025]
13.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR' for medical statistics. Bone Marrow Transplant. 2013; 48: 452-8.
14.Statistics Canada. Focus on Geography Series, 2021 Census of Population, British Columbia, Province. 2022. https://www12.statcan.gc.ca/census-recensement/2021/as-sa/fogs-spg/page.cfm?lang=E&topic=10&dguid=2021A000259 [Accessed: 29 January 2025]
15.Paulson K, Brazauskas R, Khera N, He N, Majhail N, Akpek G, et al. Inferior Access to Allogeneic Transplant in Disadvantaged Populations: A Center for International Blood and Marrow Transplant Research Analysis. Biol Blood Marrow Transplant. 2019; 25: 2086-90.
16.Bonikowska A, Morissette R, Schellenberg G. Cumulative earnings of Black, Chinese, South Asian and White individuals born in Canada. 2024; https://www150.statcan.gc.ca/n1/pub/36-28-0001/2024011/article/00004-eng.htm [Accessed: 29 January 2025]
17.Mielcarek M, Gooley T, Martin PJ, Chauncey TR, Young BA, Storb R, et al. Effects of race on survival after stem cell transplantation. Biol Blood Marrow Transplant. 2005; 11: 231-9.
Search
News