Volume 8 (2025) Issue 3 No.5 Pages 244-249
Abstract
Background: Chimeric antigen receptor T-cell (CAR-T) therapy has transformed the treatment landscape for relapsed or refractory non-Hodgkin lymphoma, achieving a 5-year overall survival rate of 40-50%. However, relapse remains a major challenge, especially due to CD19-negative clones. Epcoritamab, a bispecific antibody targeting CD20 and CD3, offers a potential solution for post-CAR-T relapse; however, clinical data in this setting remain limited, particularly in Japan.
The case: A 68-year-old woman with CD5-positive diffuse large B-cell lymphoma (DLBCL) relapsed multiple times following various treatments, including CAR-T therapy. Genetic profiling revealed a MYD88/CD79B-mutated (MCD) subtype with CD19-negative clones resistant to CAR-T cells. Given her high tumor burden, she received debulking therapy followed by epcoritamab treatment. Despite concerns about tumor lysis syndrome, cytokine release syndrome, and neurotoxicity, no significant adverse events occurred. The patient achieved a complete remission, demonstrating the efficacy and safety of this approach.
Conclusion: This case highlights the potential of epcoritamab combined with debulking therapy for treating CAR-T-refractory CD19-negative DLBCL. This is the first validated case in Japan showing that epcoritamab is a viable and safe treatment option for post-CAR-T relapse, addressing a critical unmet need in the current therapeutic landscape.
Introduction
The five-year overall survival (OS) rate following chimeric antigen receptor T-cell (CAR-T) therapy is approximately 40-50%, with half of patients achieving a potential cure1. However, relapse remains a significant unmet clinical need1. Recently, epcoritamab, a bispecific antibody (BsAb) targeting CD20 and CD3, has recently emerged as a promising therapeutic option, potentially representing a paradigm shift in the treatment of relapsed or refractory non-Hodgkin lymphoma (NHL)2–4. International phase 1/2 trials (n=157) demonstrated epcoritamab's efficacy in relapsed NHL, showing an overall response (OR) rate of 63% (complete response [CR] 38%; partial response [PR] 24%) and a 1-year OS rate of approximately 50%2–4. In a subgroup analysis of the pivotal study, the CR rate among patients relapsing after CAR-T therapy (n=61) was 34.4% (95% Confidence Interval (CI), 22.7-47.7%)2–4. Despite these promising results, detailed clinical data on the long-term efficacy and safety of epcoritamab in post-CAR-T patients remain limited2–4. Furthermore, the domestic phase 2 trial in Japan have not included post-CAR-T relapsed patients, leaving the efficacy of epcoritamab in this context uncertain within the Japanese population5.
Here, we present the first validated case in Japan demonstrating the effective and safe use of epcoritamab for relapsed CD5+CD19– diffuse large B-cell lymphoma (DLBCL) following CAR-T therapy.
Case Report
A 68-year-old woman was diagnosed with CD5+ DLBCL in 2017 and initially received R-EPOCH (Rituximab, Etoposide, Prednisone, Vincristine, Cyclophosphamide, Doxorubicin) therapy due to its poor prognosis6. At first relapse, she was treated with R-ESHAP (Rituximab, Etoposide, Methylprednisolone, Cytarabine, Cisplatin). Upon second relapse, salvage therapy followed by autologous peripheral blood stem cell transplantation (auto-PBSCT) was administered. During her third relapse, she presented with poor performance status (PS), severe organ dysfunction, and central nervous system (CNS) infiltration, which were managed with high-dose methotrexate (MTX) and subsequent CAR-T therapy (tisagen lecleucel), achieving CR.
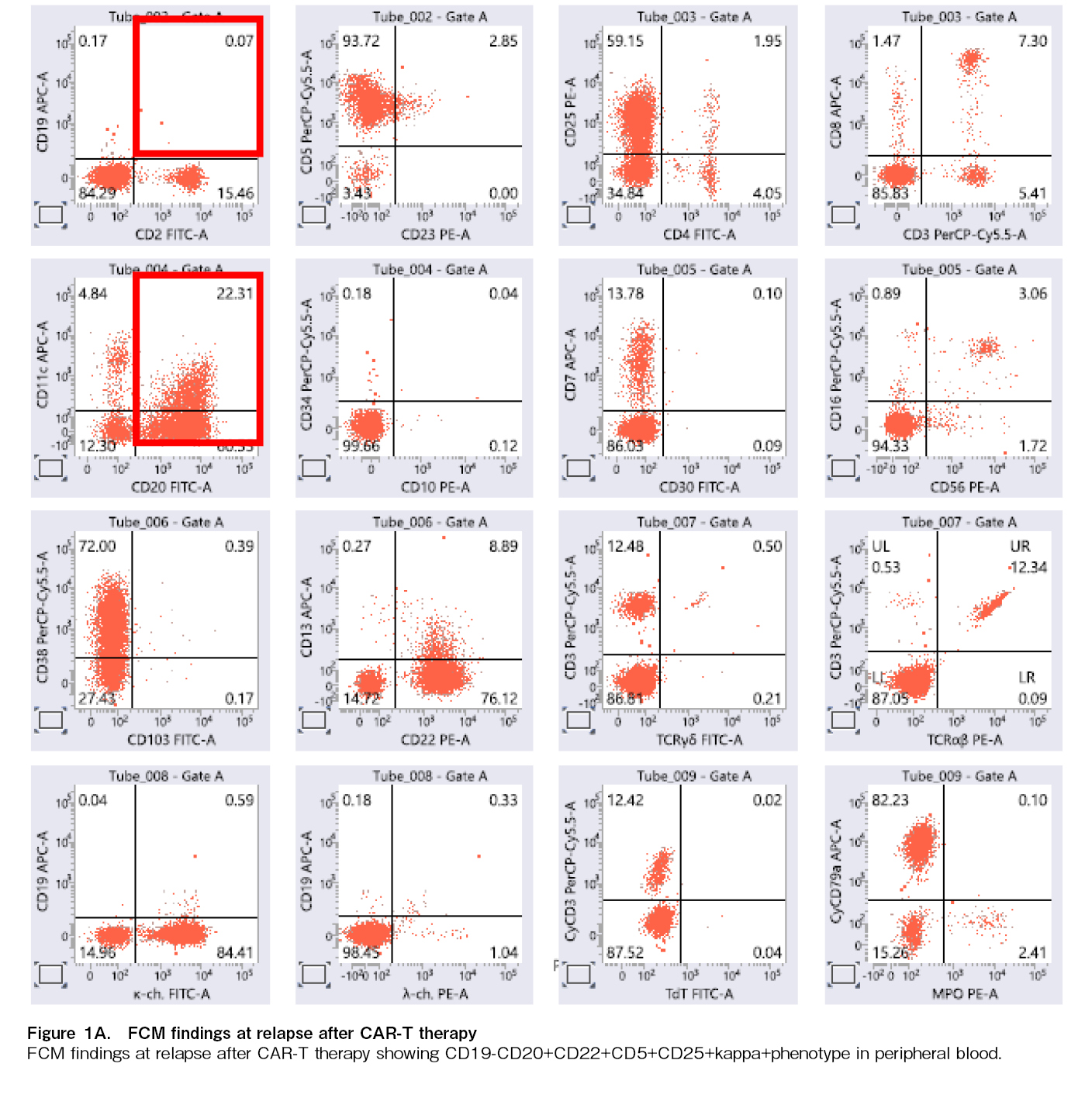
However, three years and four months after CAR-T therapy, the patient relapsed with a CD19-negative clone, resistant to CAR-T cells. Flow cytometry analysis of peripheral blood revealed a CD5+CD19–
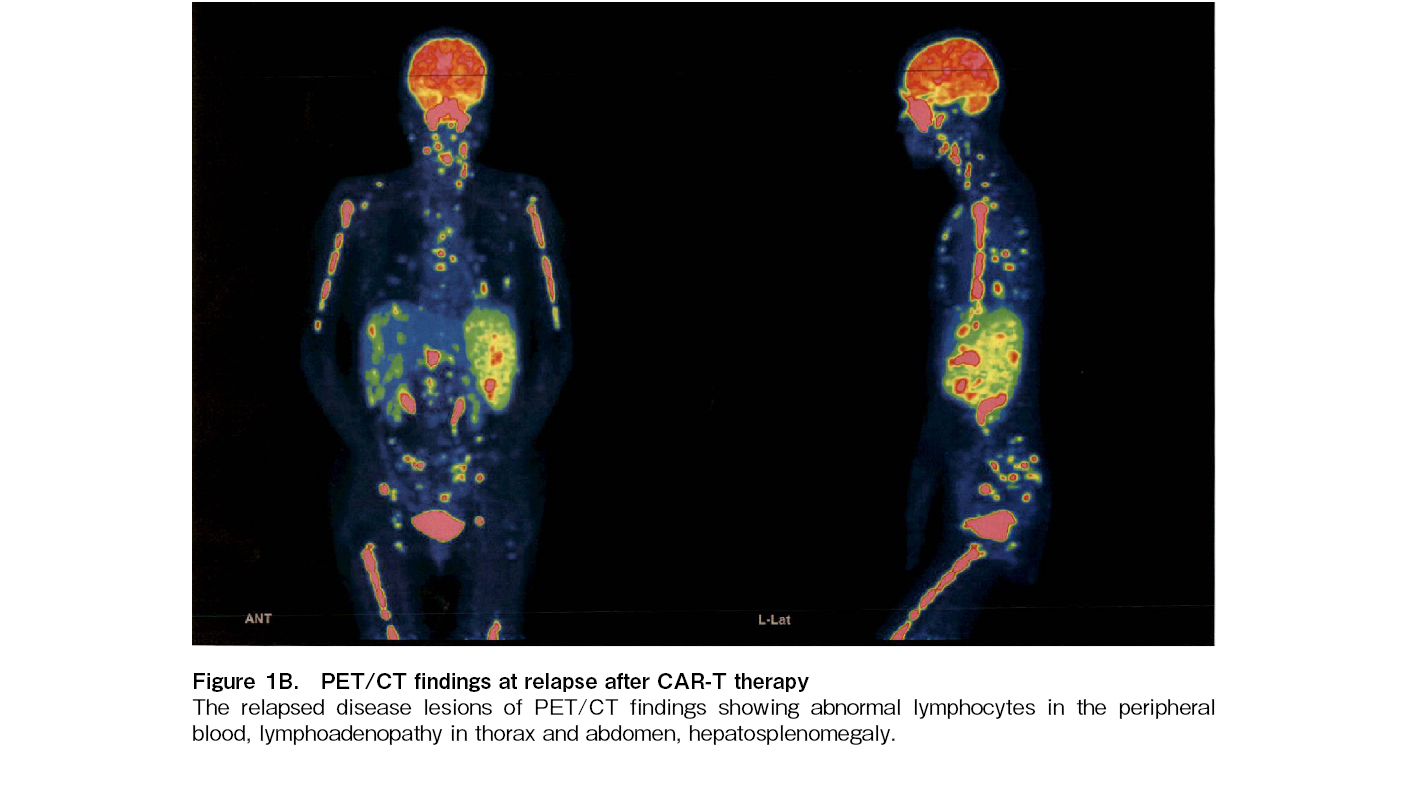
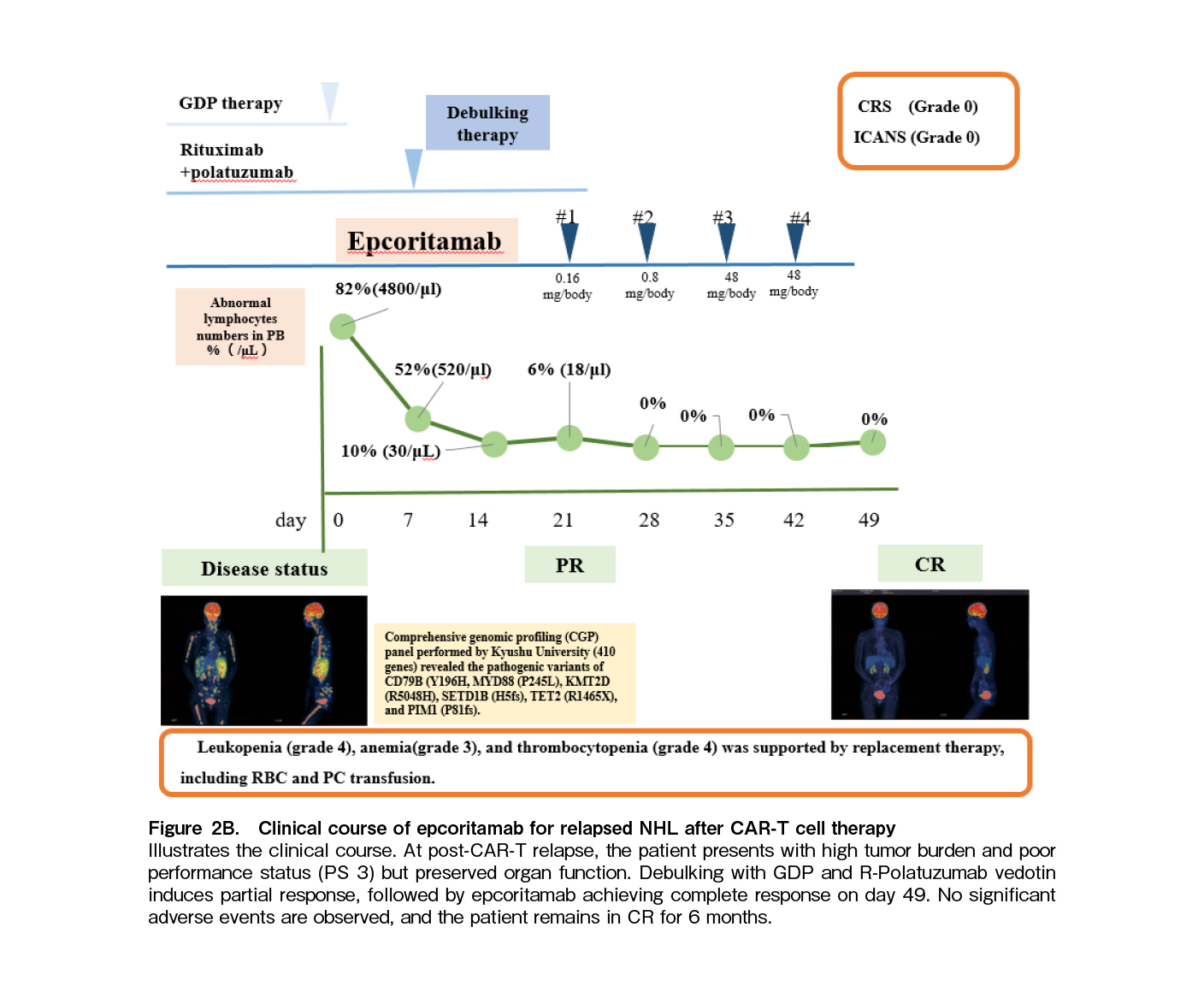
The patient's clinical course is illustrated in Figure 2B. At the time of post-CAR-T relapse, the patient presented with a high tumor burden and poor PS 3, yet their organ function remained preserved. Given the high tumor burden at relapse post-CAR-T therapy, debulking treatment with GDP (Gemcitabine, Dexamethasone, Cisplatin) and R-Polatuzumab vedotin was initiated. Following the administration of debulking therapy, the patient demonstrated a partial response (PR), as shown in Figure 2B. This was followed by epcoritamab, a BsAb targeting CD20 and CD3, leading to a CR again on day 49. Despite potential risks of tumor lysis syndrome (TLS), cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), and immune effector cell-associated hematotoxicity (ICAHT), no significant adverse events occurred. Thus, the patient remained in CR without disease progression for 6 months.
Discussion
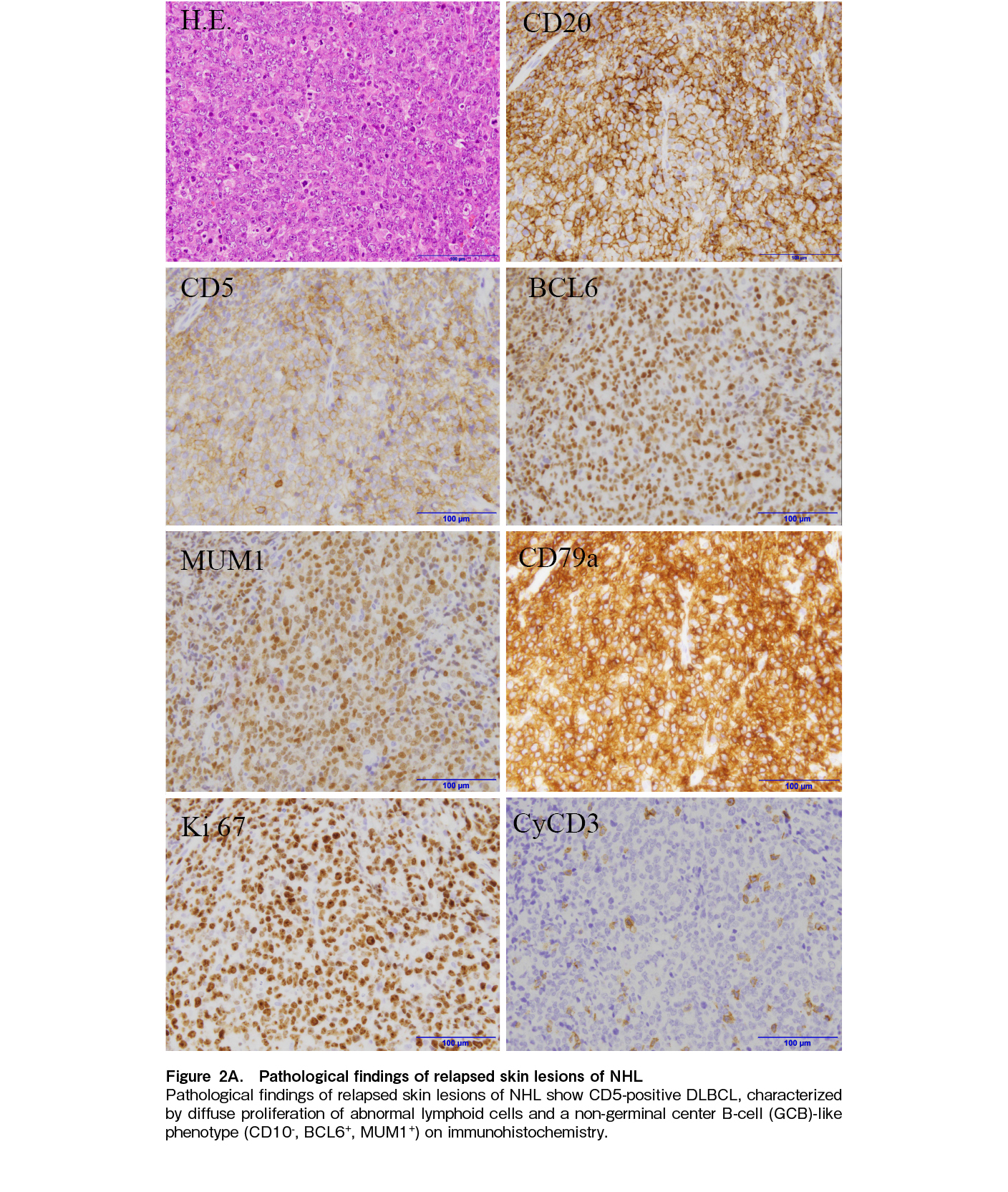
We reported a DLBCL case of MCD subtype relapsed after CAR-T therapy with CD19-negative clone. The patient was successfully treated with debulking cytotoxic agents followed by epcoritamab with no significant adverse events. This case suggested a promising therapeutic strategy in the very challenging clinical setting of CAR-T-refractory lymphoma. Furthermore, a detailed explanation of a patient's clinical course is of value, as many physicians still lack experience with such treatment. Notably, the incorporation of comprehensive genomic profiling data enhances the value of the present report.
Genetic abnormalities identified in our case were consistent with the MCD subtype, characterized by mutations of MYD88 and CD79B, which is associated with poor prognosis, with a five-year OS rate of 26%7. Additional recurrent mutations were also identified in this patient such as PIM1, SETD1B, and KMT2D7. Despite multiple relapses, the patients achieved a durable CR of 40 months following CAR-T therapy, highlighting the potential effectiveness of CAR-T therapy. Notably, a recent study found no significant difference in CR rates (
Epcoritamab, a BsAb targeting CD20 and CD3, offers a promising option for patients with CD19-negative relapses after CAR-T therapy2–4, 10. Although data on debulking prior to epcoritamab are limited, Thieblemont et al. reported higher response rates in patients with low tumor burden (< 80 cm3) compared to high tumor burden (OR rate: 67% vs 52%; CR: 52% vs 32%)4. To optimize the efficacy of epcoritamab, we administered GDP and R-polatuzumab vedotin as debulking therapies11 before epcoritamab, resulting in a partial response. The present report may also underscore the significance of debulking therapy; however, the relevant previous references could not be identified through the literature search that was conducted until May 31, 2025, using PubMed, Cochrane Library, and Scopus. This approach may be feasible for patients with good general condition and preserved organ function.
Epcoritamab, like CAR-T therapy, carries risks of excessive immune-related complications, including CRS, ICANS, and ICAHT1. In previous studies, grade ≥ 3 neutropenia occurred in 16.6%, anemia in 12.1%, and thrombocytopenia in 5.1% of patients2–4. Our patient experienced no such hematologic complications, possibly due to the extended interval since CAR-T therapy. Effective management of cytopenia and prevention of infectious complications are crucial during epcoritamab treatment, especially among patients with short interval from CAR-T therapy.
In conclusion, this is the first reported Japanese case demonstrating the efficacy and safety of epcoritamab in relapsed DLBCL post-CAR-T therapy, with tolerable adverse events. Combining epcoritamab with cytotoxic agents may be a viable strategy for CAR-T-refractory patients with high tumor burden. Further studies are warranted to refine the role of BsAbs in this clinical setting.
Acknowledgments
We thank the medical and nursing staff who cared for the patient.
Informed Consent
The informed consent was obtained from the patient in our case report.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
Acknowledgments
We thank the medical and nursing staff who cared for the patient.
Author Contributions
TS, NK, YS, YM and KT have contributed equally to this manuscript. TY, KM, KM, MS, MO, RI, SS, TN, KY, KM, FJ, TS and IK cared for the patient. FJ, TS, KM, KM, KO, KK, KK, and KA supervised the case presentation and discussion. ST, KN, SY, MY, and TK are equally contributed authors.
References
1.Westin J, Sehn LH. CAR T cells as a second-line therapy for large B-cell lymphoma: a paradigm shift?. Blood. 2022; 139: 2737-46.
2.Thieblemont C, Phillips T, Ghesquieres H, Cheah CY, Clausen MR, Cunningham D, et al. Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J Clin Oncol. 2023; 41: 2238-47.
3.Thieblemont C, Karimi YH, Ghesquieres H, Cheah CY, Clausen MR, Cunningham D, et al. Epcoritamab in relapsed/refractory large B-cell lymphoma: 2-year follow-up from the pivotal EPCORE NHL-1 trial. Leukemia. 2024; 38: 2653-62.
4.EHA Library, Thieblemont C, Karimi Y, Ghesquieres H, Cheah C, Clausen MR, et al. EXTENDED FOLLOW-UP BEYOND 2.5 YEARS SHOWS LONG-TERM EFFICACY IN COMPLETE RESPONDERS FOLLOWING EPCORITAMAB MONOTHERAPY IN RELAPSED OR REFRACTORY LARGE B-CELL LYMPHOMA. 2024. https://library.ehaweb.org/eha/2024/eha2024-congress/419238 [Accessed: 23 December 2024]
5.Izutsu K, Kumode T, Yuda J, Nagai H, Mishima Y, Suehiro Y, et al. Subcutaneous epcoritamab monotherapy in Japanese adults with relapsed/refractory diffuse large B-cell lymphoma. Cancer Sci. 2023; 114: 4643-53.
6.Nato Y, Miyazaki K, Maruyama D, Takahashi H, Sunami K, Murakami S, et al. Treatments and Outcomes of Newly Diagnosed CD5-Positive Diffuse Large B-Cell Lymphoma: A Multi-Institutional Observational Study. Hematol Oncol. 2025; 43: e70047.
7.Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med. 2018; 378: 1396-407.
8.Shi H, Zheng P, Liu R, Xu T, Yang F, Feng S, et al. Genetic landscapes and curative effect of CAR T-cell immunotherapy in patients with relapsed or refractory DLBCL. Blood Adv. 2023; 7: 1070-5.
9.Goto H. Mechanisms and Clinical Features of Relapse and/or Refractory to CAR-T-cell Therapy. Japanese Journal of Translantaion and Cellular therapy. 2023; 12: 172-80. (in Japanese)
10.Bayly-McCredie E, Treisman M, Fiorenza S. Safety and Efficacy of Bispecific Antibodies in Adults with Large B-Cell Lymphomas: A Systematic Review of Clinical Trial Data. Int J Mol Sci. 2024; 25: 9736.
11.Gouni S, Rosenthal AC, Crombie JL, Ip A, Kamdar MK, Hess B, et al. A multicenter retrospective study of polatuzumab vedotin in patients with large B-cell lymphoma after CAR T-cell therapy. Blood Adv. 2022; 6: 2757-62.
Search
News