Volume 8 (2025) Issue 3 No.1 Pages 217-224
Abstract
Acute graft-versus-host disease (aGVHD) is a life-threatening complication that can develop after allogeneic hematopoietic stem cell transplantation. Patients with steroid-refractory aGVHD (SR-aGVHD) have an extremely poor prognosis. Ruxolitinib is an approved treatment for SR-aGVHD. However, there is a paucity of real-world data on the clinical outcomes of patients with SR-aGVHD treated with ruxolitinib.
We conducted a retrospective analysis using hospital records of the clinical outcomes of patients who underwent stem cell transplantation at our center between January 2021 and December 2022 and developed steroid-refractory aGVHD which was treated with ruxolitinib.
During the study period, 381 patients underwent allogeneic stem cell transplantation at our center. Amongst these, 160 (42.0%) developed aGVHD. Of these, 59 (36.8%) had SR-aGVHD and 40 were treated with ruxolitinib. Ruxolitinib therapy was administered after a median of 6 days (range 3-29) from onset of aGVHD. Amongst the 28 patients who survived at day 28 (12 died before the day 28 response could be assessed), a total of 16 patients (57.1%) attained a response (complete response (n=12) or partial response (n=4)). Infectious complications were the most common adverse event (n=39; 97.5%), followed by severe cytopenia (grades 3 to 4) in 25 (62.5%) patients. The median follow-up of the cohort was 5 months (range 1 to 29 months). At the last follow up, 30 (75%) patients died; 3 patients died of progression of steroid-refractory aGVHD, 14 died of progression of aGVHD with infection, 10 died of underlying infection and 3 had disease relapse.
From our real-world analysis, we conclude that though the outcomes in patients with SR-aGVHD responding to ruxolitinib are encouraging, there is still a large unmet need for novel strategies for improving outcomes and reducing infection-related mortality, even while there is access to ruxolitinib.
Introduction
Acute graft-versus-host disease (aGVHD) remains an important complication following allogeneic hematopoietic stem cell transplantation (HSCT) in the modern era. With matched related and unrelated donors, the cumulative incidence of aGVHD remains 40-60%1. Systemic steroid therapy is the standard first-line treatment for aGVHD2,3. However, in ~35-50% of patients, aGVHD becomes refractory to systemic steroid therapy4,5. It is one of the most serious complications of HSCT, and its timely recognition and treatment are key elements of a successful outcome6. Many agents have been evaluated as second-line treatments for aGVHD7. The morbidity and mortality are high in these patients. The average 6-month survival estimate across 25 studies was 49%7 and another study reported an estimated 2-year survival rate of 17%8 amongst patients with steroid-refractory aGVHD (SR-aGVHD) prior to the FDA approval and widespread availability of ruxolitinib.
Ruxolitinib is an Food and Drug Administration (FDA) approved drug for SR-aGVHD9. Ruxolitinib therapy led to significant improvements in efficacy outcomes amongst patients with SR-aGVHD, as was demonstrated by the REACH 2 trial, which led to its approval as a second-line agent9. There is limited real-world data on the use of ruxolitinib for treatment of SR-aGVHD10,11, especially from developing countries with high rates of infections and infection-related mortality during transplantation.
Materials and Methods
After Institutional Review Board approval (IRB No. 15855, dated October 25, 2023), a retrospective study analyzing the data of patients with SR-aGVHD who underwent HSCT at Christian Medical College and Hospital, Vellore, from January 2021 to December 2022 was conducted with a waiver of informed consent. Hospital records were used to retrieve the following data: patient demographics, transplant-related characteristics, aGVHD characteristics (including diagnosis, grade, organs involved at diagnosis, and maximum grade), treatments received for aGVHD, timing and dose of ruxolitinib, and clinical outcomes (including aGVHD recurrence, rate of infections – viral, bacterial, mycobacterial, fungal, and parasitic, and all-cause mortality) in patients who were refractory to or dependent on systemic corticosteroids.
All patients who underwent Human Leukocyte Antigen (HLA) full-matched donor transplant were started on GVHD prophylaxis with short course methotrexate, along with calcineurin inhibitor and anti-thymocyte globulin (ATG) for unrelated donor transplants. In case of toxicity due to calcineurin inhibitors, sirolimus (n=8) or mycophenolate mofetil (MMF) (n=1) were used. All patients who underwent haploidentical transplant were started on GVHD prophylaxis with triple immunosuppression, which consisted of cyclophosphamide, MMF, and a calcineurin inhibitor (either cyclosporine or tacrolimus). In case of toxicity due to calcineurin inhibitors, sirolimus (n=3) was used.
Antimicrobial prophylaxis consisted of fluconazole and acyclovir starting on day 1 post-transplant. Oral penicillin and sulfamethoxazole-trimethoprim as prophylaxis were added after recovery of blood counts. In patients who developed aGVHD and received intravenous corticosteroids, antifungal prophylaxis was switched to oral posaconazole. For patients developing fever on immunosuppressive therapy post-transplant, the first line of antibiotics used was a combination of cefoperazone/sulbactam, along with amikacin (in case of febrile neutropenia). All patients with prolonged fever despite adequate antibiotics underwent high-resolution computed tomography of the chest and, if suspicious for fungal infection, underwent serum galactomannan testing. All patients were routinely monitored for cytomegalovirus (CMV) infection once a week after engraftment using a DNA polymerase chain reaction (PCR) from peripheral blood. If there was CMV reactivation (defined as > 1,000 copies/mL in blood), patients were started on intravenous ganciclovir in the absence of cytopenias. BK virus (BKV) was routinely monitored using a quantitative PCR from the urine, once a week after engraftment. Patients who had symptomatic BK infection were treated with cidofovir.
aGVHD was diagnosed by the treating physician considering clinical and laboratory findings. The severity of aGVHD was graded according to modified Glucksberg criteria3. SR-aGVHD included all patients who had progression after 3-5 days or failure to improve in any organ after 5-7 days of therapy onset with steroids. The initial dose of ruxolitinib was not uniform amongst the study population, ranging from 2.5-5 mg once or twice daily for children below 12 years of age and 5-10 mg twice daily for adults as per the clinician's discretion.
Ruxolitinib – definitions of response
Response to ruxolitinib (RUX) treatment was defined as follows: (1) complete response (CR)―absence of all aGVHD manifestations; (2) partial response (PR)―significant improvement (at least one grade lower) in all initially affected organs. All other types of responses were considered as treatment failures10.
Statistics
Descriptive statistics were used, such as mean (standard deviation) and median (range) for clinical and laboratory data. For categorical variables, numbers and percentages were used. The chi-square test and Fisher's exact test were used to find the association between two categorical variables. The independent t-test and Mann-Whitney U test were used for continuous variables. Survival analysis was performed using the Kaplan Meier method. Univariate logistic regression analysis was performed for evaluating predictors of response. A p-value < 0.05 was regarded as statistically significant. All analysis was performed using SPSS version 23.0 (SPSS Inc, Chicago, IL).
Results
During the study period from January 2021 to December 2022, a total of 381 patients underwent HSCT, amongst which 245 patients underwent matched unrelated donor (MUD)/matched sibling donor (MSD)/matched related donor (MRD) transplant and 136 patients underwent haploidentical stem cell transplant. Amongst the 381 patients who underwent HSCT from January 2021 to December 2022, a total of 160 patients (42%) developed aGVHD. Amongst the patients who underwent MUD/MSD/MRD transplant, 100 (out of 245 patients; 40.8%), developed aGVHD. Sixty patients (out of 136; 44.1%) who underwent haploidentical stem cell transplant, developed aGVHD. Amongst the 100 patients who developed aGVHD following MUD/MSD/MRD transplant, 38 patients (38%) developed SR-aGVHD. Amongst the 60 patients who developed aGVHD following haploidentical transplant, 21 patients (35%) developed SR-aGVHD. Overall, 59 (36.8%) of the patients who developed aGVHD were steroid refractory.
Amongst the 59 patients who developed SR-aGVHD, 40 patients were treated with ruxolitinib as a salvage therapy for SR-aGVHD. The median age at transplant was 24 years (range 1.5-62 years). The indications for transplant included both malignant and non-malignant hematological disorders. Study patients received allografts for severe aplastic anemia (n=10), acute myeloid leukemia (n=8), myelodysplastic syndromes (n=5), acute lymphoblastic leukemia (n=3), beta thalassemia major (n=3), Fanconi anemia (n=3), chronic myeloid leukemia (n=2), primary myelofibrosis (n=1), and others (n=5). The other indications for transplant included relapsed refractory natural killer T-cell lymphoma (n=1), diffuse large B-cell lymphoma (n=1), severe congenital neutropenia (n=1), combined immunodeficiency with RAG1 gene mutation (n=1), leukocyte adhesion defect (n=1), and Shwachman-Diamond syndrome (n=1). All patients with malignant disease were in morphologic complete remission at the time of transplant. Fifteen out of these 16 patients were also MRD negative, except for one patient who was MRD positive (Acute Myeloid Leukemia with MRD 0.345) at the time of transplantation. The baseline characteristics are shown in Table 1.
Twenty-three patients (57.5%) received stem cells from HLA-matched donors (either related or unrelated). One patient was transplanted from a 9/10 HLA-matched unrelated donor and 16 (40%) received haploidentical transplantation. In total, 28 patients (70%) received reduced-intensity conditioning (RIC), whereas myeloablative conditioning (MAC) was provided for 6 (15%) subjects. Six patients (15%) received non-myeloablative conditioning (NMA). Peripheral blood was a source of stem cells for all transplanted patients.
Three patients (7.5%) developed grade II, two patients (5%) had grade III, and 35 (87.5%) had grade IV aGVHD. Fourteen patients (35%) had involvement of a single organ (most commonly the gut), 18 patients (45%) had involvement of two organs (most common being the gut and liver), and 8 patients (20%) had involvement of three organs (most common being the gut, liver, and skin). The most common organ system involved was the gut, seen in 38 patients (95%). The second most common organ system involved was the liver, which was seen in 23 patients (57.5%), followed by the involvement of skin in 17 patients (42.5%). The median time from allogeneic HSCT to aGVHD occurrence was 32 days (range 12-155). The GVHD characteristics are summarized below in Table 2.
First-line treatment consisted of methylprednisolone at the maximum dose of 2 mg/kg/day in all patients except in 13 patients, who received a maximum steroid dose of 1 mg/kg/day. Ruxolitinib therapy was administered a median of 6 days after prior immunosuppressive therapies (range 3-29). All patients who received ruxolitinib also were continued on their GVHD prophylaxis (primarily consisting of a calcineurin inhibitor +/- MMF), except in 4 patients who received ruxolitinib along with an mTOR inhibitor (sirolimus), the doses of which were titrated according to clinical response and biochemical levels. The initial dose of ruxolitinib was variable amongst the patient group, ranging from 2.5 mg every alternate day to 10 mg twice daily as follows: 2.5 mg on alternate days (1 patient; 2.5%), 2.5 mg once daily (2 patients; 5%), 2.5 mg twice daily (3 patients; 7.5%), 5 mg once daily (9 patients; 22.5%), 5 mg twice daily (13 patients; 32.5%), 10 mg once daily (2 patients; 5%) and 10 mg twice daily (10 patients; 25%). If no limiting toxicities occurred, the dose was escalated based on the clinical condition and response. However, 3 patients required ruxolitinib interruption during therapy due to severe neutropenia.
Amongst 40 patients, 32 received ruxolitinib as second-line therapy at a median time of 5 days (interquartile range of 3 to 7 days; range 3 to 18 days) from the initiation of steroids. Of the remaining 8 patients, the second-line therapy was etanercept +/- basiliximab (n=7) and MMF (n=1) which was started at a median of 5.5 days (interquartile range 5 to 7 days; range 3 to 11 days) following steroids. In these 8 patients, ruxolitinib was started as the third-line therapy after a median of 10 days (range 5 to 18 days) beyond the initiation of the second line.
In our cohort, another line of immunosuppression other than steroids, calcineurin inhibitors, and ruxolitinib was used in 29 patients (72.5%). Of these, 8 patients had received ruxolitinib as the third-line therapy following a second-line immunosuppressant. Of the remaining 32 patients who received second-line ruxolitinib, 21 patients received a third-line therapy. The reasons for initiation of the third line were GVHD progression (n=10), ruxolitinib intolerance (n=1), and physician discretion (n=10). The most used additional line agents were etanercept (26 patients, 65%) and basiliximab (7 patients, 16.3%). Amongst the patients who required third-line therapy or beyond for SR-aGVHD, 6 patients received only etanercept, 2 patients received only basiliximab, 20 patients received etanercept along with basiliximab. One patient received plasma exchange (along with sirolimus and etanercept) for control of steroid-refractory acute liver GVHD, and one patient required extracorporeal photopheresis (along with etanercept).
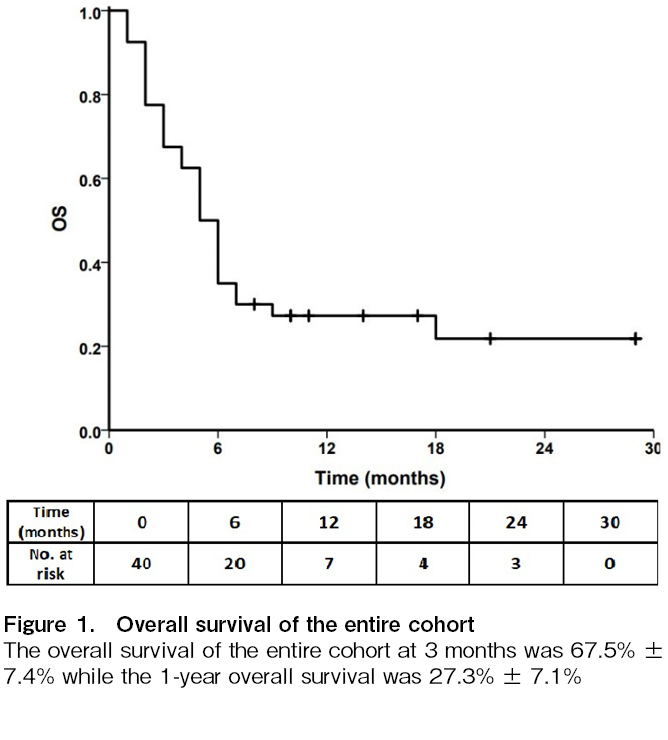
The overall survival at 3 months was 67.5% ± 7.4% while the 1-year overall survival was 27.3% ± 7.1%, as shown in Figure 1. Amongst the 28 patients who survived at day 28 (12 died before the day 28 response could be assessed, with the cause of death being progressive GVHD with sepsis in 8, progressive GVHD and massive hematochezia in 1, bacterial sepsis in 1, fungal brain abscess in 1, and cellulitis in 1), a total of 16 patients (57.1%) had a response (CR (n=12; 42.8%) or PR (n=4; 14.2%)), as summarized in
Using logistic regression and the Mann-Whitney test, no statistically significant correlation was noted between donor type, indication for transplant, day of onset of GVHD, maximum grade of GVHD, number or organs involved, day of initiation of ruxolitinib, and response to ruxolitinib (
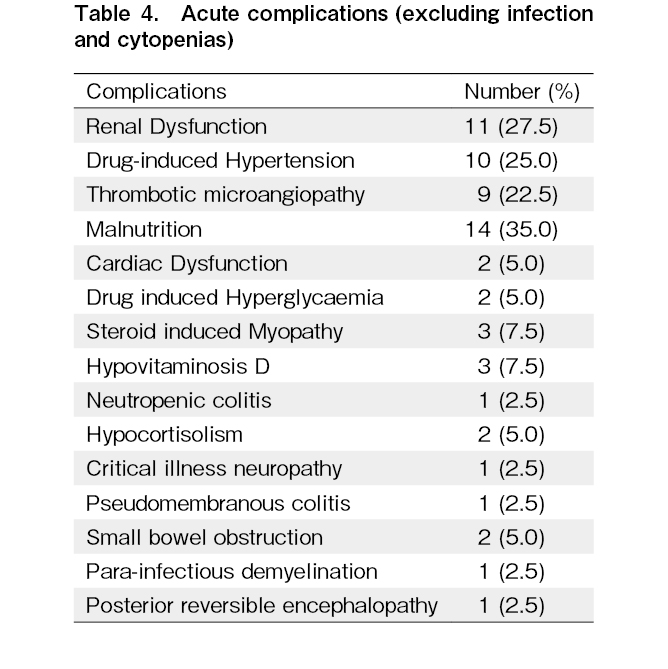
The median follow-up from the occurrence of aGVHD was 5 months (range 1 to 29 months; 15.5 months amongst survivors). At the last follow up, 30 (75%) patients had died. The main cause of death was progression of aGVHD with sepsis (14 patients; 46.6%) and its complications including septic shock, acute respiratory distress syndrome (ARDS), and multiple organ dysfunction syndrome (MODS), followed by infection alone (10 patients; 33.3%). The other causes included progression of refractory aGVHD (3 patients; 10%) and relapse (3 patients; 10%). Infections (97.5%), followed by severe cytopenia (including both neutropenia and thrombocytopenia, grades 3 to 4) in 25 (62.5%) patients, were the major adverse events after the initiation of ruxolitinib therapy. Thirty-five patients developed CMV reactivation, and polyoma BKV reactivation was noted in 8 individuals. Two patients with CMV reactivation developed CMV colitis. Twenty-five patients had culture-positive bacteriemia (Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii, coagulase-negative Staphylococcus, and others). Out of 40 patients, 21 developed invasive fungal infection (fungal pneumonia (n=12), fungemia (n=4), fungal brain abscess/meningoencephalitis (n=3), fungal sinusitis (n=1), and fungal liver abscess (n=1)). Prophylactic antifungals were used in 39 patients at the initiation of steroids (20 received posaconazole without drug level monitoring, 10 received amphotericin, 4 received anidulafungin, 3 received voriconazole, while 2 received isavuconazole as prophylaxis). Clostridium difficile infection was seen in 18 patients. No patient developed tuberculosis. The spectrum of infections is shown in Table 3. Amongst the cytopenia, thrombocytopenia (grade 3-4, < 50,000/mm3) was seen in 13 patients, neutropenia (grade 3-4, absolute neutrophil count < 1,000 cells/mm3) in 11 patients, and anemia requiring transfusion in 1 patient. The other acute complications noted are enumerated in Table 4.
The financial burden and average drug cost amongst the two groups (MUD/MRD/MSD versus haploidentical stem cell transplant) were compared. The average total expense for patients who underwent MUD/MRD/MSD transplant from the onset of aGVHD till last follow-up was Indian rupee (INR) 17,61,005/- (INR 1,44,347 – 64,41,035) as compared to INR 25,33,125/- (INR 1,43,114 – 46,68,169) in the haploidentical group. Amongst the total cost, the average drug cost was INR 7,48,329/- (INR 1,03,804 – 18,89,767) in the MUD/MRD/MSD group as compared to INR 11,72,873/- (INR 1,61,767 – 27,55,075) in the haploidentical group.
Discussion
HSCT is a curative treatment for numerous hematologic disorders12. The therapeutic benefits of HSCT in malignancies are primarily derived from an anti-leukemia effect (graft-versus-leukemia effect) that is mediated by donor T-cells present in the graft. Unfortunately, these donor T-cells also induce GVHD, the major life-threatening complication of HSCT13.
An effective therapy for advanced (grade III-IV) aGVHD still remains a challenge. High-dose corticosteroids (CS) are accepted as the first-line treatment. It has been estimated that nearly half of graft recipients suffering from advanced aGVHD respond to initial therapy with CS3. The absence of response to CS results in an extremely short survival of approximately 40% at 6 months7. The agents commonly used as second and further lines of therapy for SR-aGVHD include ATG, mycophenolate mofetil, calcineurin inhibitors, or extracorporeal photopheresis; however, the response is mixed3. In 2019, FDA approved ruxolitinib, a JAK1/2 inhibitor, for therapy of SR-aGVHD in adult and pediatric patients > 12 years14. Approval was based on favorable results of the REACH2 clinical trial in which ruxolitinib showed an advantage over other immunosuppressive therapies. The response rate with ruxolitinib at day 28 was 62.3% compared with 39.4% in the control group (p < 0.001)9. Ruxolitinib is reported to block dendritic cell activation, reduce the migration of neutrophils into GVHD affected organs, and limit T-cell proliferation15.
Previous studies appear to confirm the beneficial effect of ruxolitinib in SR-aGVHD, amongst which the most pivotal studies included REACH1 and REACH2. REACH1 was an open-label phase 2 study, which included patients aged at least 12 years with grades II to IV steroid-refractory aGVHD, who received ruxolitinib. At day 28, 39 patients (54.9%) had an overall response, including 19 (26.8%) with CR. The best overall response rate was 73.2% (CR 56.3%), with a median duration of response of 345 days. The overall survival estimate at 6 months was 51.0%9. The REACH2 study showed an overall response at day 28 higher in the ruxolitinib group than in the control group (62% [96 patients] vs. 39% [61 patients]; p < 0.001). In our real-world cohort, the overall response rate at 28 days was 57% (16 of 28 evaluable patients).
Nineteen patients with grade III/IV SR-aGVHD were analyzed by Abedin S et al., who reported a response rate of 84% at day 28 with a 6-month overall survival (OS) of 58%16. Amongst responders, 9 patients achieved CR and 7 patients PR. However, 38 of our patients had grade IV disease as compared to only 21% in the above study. Similarly, another analysis on 23 Chinese patients with SR-aGVHD reported an almost 87% overall response rate for ruxolitinib treatment with a 1-year OS of 82%17. However, this study had patients with grade II aGVHD, which accounted for ~40% of the entire cohort and in none of the patients more than 2 organs were involved as compared to our study where 87.5% of our patients had grade IV aGVHD, 18 patients (45%) had involvement of two organs (most common being the gut and liver), and 8 patients had involvement of three organs (most common being the gut, liver, and skin). Of note is that ruxolitinib was initiated after a median of 5 days of GVHD duration, similar to our study where ruxolitinib was administered after a median of 6 days after inefficient immunosuppressive therapies (range 3-29 days).
In the REACH2 study, the most common adverse events up to day 28 were thrombocytopenia (33% in the ruxolitinib group and 18% in the control group), anemia (in 30% and 28%, respectively), and cytomegalovirus infection (in 26% and 21%, respectively)9. Similarly, in a study conducted on 18 patients who received ruxolitinib for SR-aGVHD by Spalek A et al., severe cytopenia (grades 3 to 4) was the major adverse event after the initiation of RUX therapy and occurred in 89% of patients. Thrombocytopenia (n=11; 61%) and neutropenia (n=4; 22%) were common; however, severe anemia was demonstrated in only one patient11. In this study, it was also noted that there was no significant correlation between cytopenia and ruxolitinib efficacy. In our study, the most common adverse events recorded were infections seen in 39 patients [97.5%] and cytopenia seen in 25 [62.5%] of the patients. As was seen in the REACH2 trial, the most prevalent infection was CMV reactivation, as in our study, which was noted in 35 of the 40 patients.
In the REACH2 trial9, 47% in the ruxolitinib group and 51% in the control group had died by the data cutoff date. Most deaths were attributed to aGVHD (22% in the ruxolitinib group and 25% in the control group). Other causes of death were underlying disease progression, including neoplasms (in 8 patients in the ruxolitinib group and 8 in the control group), multiple organ dysfunction syndrome (in 3 and 1 patient, respectively), sepsis (in 4 and 3 patients, respectively), and septic shock (in 3 and 3 patients, respectively). In a study by Spalek A et al., after a median follow-up of 55 days (range 29-706), 14 patients (78%) died, mainly due to further progression of GVHD11. In the present study at the last follow up, 30 (75%) patients had died. Causes of death included progression of aGVHD with sepsis (14 patients; 46.6%) and its complications, including septic shock, ARDS and MODS, followed by infection alone (10 patients; 33.3%). The other causes included progression of refractory aGVHD (3 patients; 10%) and relapse (3 patients; 10%).
It has been noted that infections, as well as infection-related mortality were high in our cohort vis-a-vis other studies. Prior data from our center18, which included all patients undergoing haploidentical stem cell transplant between 2010 and June 2020, showed that more than 90% of patients had at least one documented infection, with a 44% incidence of bacterial, 71% viral, and 38% fungal infection. It was shown that factors adversely affecting survival post-haploidentical transplant included presence of a documented bacterial (p=0.000) and fungal infection (p=0.000). It was also noted in the study that incidence of infection with MDR organisms had increased from 24.7% in 2010 to 2015 to 34.4% between 2016 to 2020. Multidrug-resistant organisms included carbapenem-resistant organisms, methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. One of the major challenges, thus, is infections with MDR organisms and the mortality associated with these19.
The limitations of the study include its retrospective nature, the small sample size, varying timelines for initiation of ruxolitinib, varying doses of ruxolitinib which may have impacted the responses, and the inclusion of patients who had received an additional line of immunosuppressants along with ruxolitinib for the analysis.
Conclusions
From our real-world analysis, we conclude that the outcomes in patients with steroid-refractory aGVHD responding to ruxolitinib are encouraging. However, there is still a large unmet need for novel strategies for improving outcomes and reducing infection-related mortality in patients with steroid-refractory aGVHD, even while there is wider access to ruxolitinib.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
Author Contributions
AR and UK conceptualized the study. AR collected data. KML performed statistical analysis. SS, SL, AJD, AK, FNA, VM, BG, and AA contributed to clinical data collection and data interpretation and reviewed the manuscript. PB contributed to data analysis and interpretation and reviewed the manuscript, and AR, UK, and BG wrote the manuscript. All authors have read and approved the current version of the manuscript.
References
1.Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012; 119: 296-307.
2.Dignan FL, Clark A, Amrolia P, Cornish J, Jackson G, Mahendra P, et al. Diagnosis and management of acute graft‐versus‐host disease. Br J Haematol. 2012; 158: 30-45.
3.Ruutu T, Gratwohl A, De Witte T, Afanasyev B, Apperley J, Bacigalupo A, et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transplant. 2014; 49: 168-73.
4.Axt L, Naumann A, Toennies J, Haen SP, Vogel W, Schneidawind D, et al. Retrospective single center analysis of outcome, risk factors and therapy in steroid refractory graft-versus-host disease after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2019; 54: 1805-14.
5.Westin JR, Saliba RM, De Lima M, Alousi A, Hosing C, Qazilbash MH, et al. Steroid-Refractory Acute GVHD: Predictors and Outcomes. Adv Hematol. 2011; 2011: 601953.
6.Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR, et al. EBMT−NIH− CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone marrow transplant. 2018; 53: 1401-15.
7.Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First-and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012; 18: 1150-63.
8.Axt L, Naumann A, Toennies J, Haen SP, Vogel W, Schneidawind D, et al. Retrospective single center analysis of outcome, risk factors and therapy in steroid refractory graft-versus-host disease after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2019; 54: 1805-14.
9.Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N Engl J Med. 2020; 382: 1800-10.
10.Murray A, Linn SM, Yu B, Novitzky-Basso I, Mattsson J, Kennah M, et al. Real-world experience with ruxolitinib therapy for steroid-refractory acute graft versus host disease. Bone Marrow Transplant. 2024; 59: 759-64.
11.Spałek A, Wieczorkiewicz-Kabut A, Koclęga A, Woźniczka K, Węglarz P, Boral K, et al. Real-world experience with ruxolitinib for steroid-refractory acute graft-versus-host disease: a single center experience. Int J Hematol. 2022; 116: 922-8.
12.Miller JS, Warren EH, van den Brink MR, Ritz J, Shlomchik WD, Murphy WJ, et al. NCI First International Workshop on The Biology, Prevention, and Treatment of Relapse After Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on the Biology Underlying Recurrence of Malignant Disease following Allogeneic HSCT: Graft-versus-Tumor/Leukemia Reaction. Biol Blood Marrow Transplant. 2010; 6: 565-860.
13.Paczesny S, Hanauer D, Sun Y, Reddy P. New perspectives on the biology of acute GVHD. Bone marrow transplant. 2010; 45: 1-11.
14.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplant. 1995; 15: 825-8.
15.Mohty M, Holler E, Jagasia M, Jenq R, Malard F, Martin P, et al. Refractory acute graft-versus-host disease: a new working definition beyond corticosteroid refractoriness. Blood. 2020; 136: 1903-6.
16.Abedin S, McKenna E, Chhabra S, Pasquini M, Shah NN, Jerkins J, et al. Efficacy, Toxicity, and Infectious Complications in Ruxolitinib-Treated Patients with Corticosteroid-Refractory Graft-versus-Host Disease after Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2019; 25: 1689-94.
17.Wei C, Zhang X, Liang D, Yang J, Du J, Yue C, et al. Ruxolitinib for Treatment of Steroid-Refractory Graft-versus-Host Disease: Real-World Data from Chinese Patients. Drug Des Devel Ther. 2021; 15: 4875-83.
18.George B, Kulkarni U, Lionel S, Devasia AJ, Aboobacker FN, Lakshmi KM, et al. Haploidentical transplantation is feasible and associated with reasonable outcomes despite major infective complications-A single center experience from India. Transplant Cell Ther. 2022; 28: 45.e1-8.
19.Bassetti M, Peghin M, Vena A, Giacobbe DR. Treatment of Infections Due to MDR Gram-Negative Bacteria. Front Med. 2019; 6: 74.
Search
News