Volume 7 (2024) Issue 2 No.2 Pages 37-40
Abstract
Secondary central nervous system (CNS) lymphomas typically require CNS-penetrating drugs; however, the available agents are limited with temporary effects and poor outcomes. Chimeric antigen receptor T (CAR-T) cell therapy (lisocabtagene maraleucel; liso-cel) has been used to treat a few cases of isolated secondary CNS lymphoma. Herein, we report the case of a 66-year-old male diagnosed with diffuse large B-cell lymphoma (Ann Arbor grade IV; R-IPI, good risk; CNS IPI: Intermediate risk) who achieved complete remission (CR) after six courses of R-CHOP therapy. Three months later, he presented with ptosis and eye movement disorder. Systemic CT and bone marrow examination revealed no lymphoma. Although cranial-enhanced MRI showed normal findings, an increased number of B-cells (51/μL) with the original lymphoma phenotype (CD19+CD79a+CD5-CD10-CD20-Igλ+) was detected in cerebrospinal fluid (CSF), indicating an isolated CNS relapse. Seven high-dose methotrexate courses led to partial response. Subsequently, the patient received CAR-T cell therapy with tolerable adverse events ― cytokine release syndrome treated with tocilizumab, no immune effector cell-associated neurotoxicity syndrome, and bone marrow failure treated with granulocyte-colony stimulating factor and eltrombopag. Sequential flow cytometry revealed a high peak of CAR-T cells and the presence of residual CAR-T cells in the peripheral blood, indicating immune surveillance of CNS lymphoma by CAR-T cells. This treatment led to a second CR. This case is the first to validate the efficacy and safety of CAR-T cell therapy for isolated secondary CNS lymphoma in clinical practice. Future accumulation of evidence on the efficacy and safety of CAR-T cell therapy is essential.
Introduction
Secondary central nervous system (CNS) lymphoma requires the penetration of chemotherapeutic agents into the CNS system. Currently, secondary CNS lymphoma poses a significant challenge due to limited drug options and unfavorable outcomes1. In recent paradigm shifts in therapeutic studies, a novel chimeric antigen receptor-T (CAR-T) cell therapy has been firmly established as an immunological treatment of activated peripheral T cells that exert an antitumor effect on the CD19 antigen by inserting a CD19 antibody and a T cell receptor chimeric gene for relapsed or refractory diffuse large B-cell lymphoma (DLBCL)2–5. Following positive outcomes from a phase 2 study (TRANSCEND NHL 001)5, lisocabtagene maraleucel (liso-cel) received approval for CAR-T cell therapy in March 20215. Furthermore, it was approved as a second-line DLBCL treatment in December 20225. However, only one Japanese case has been reported in a phase 2 trial5 where the efficacy and safety of CNS lymphomas are unknown. Herein, we report a case of CAR-T cell therapy for treating an early CNS recurrence of DLBCL.
The Case Presentation
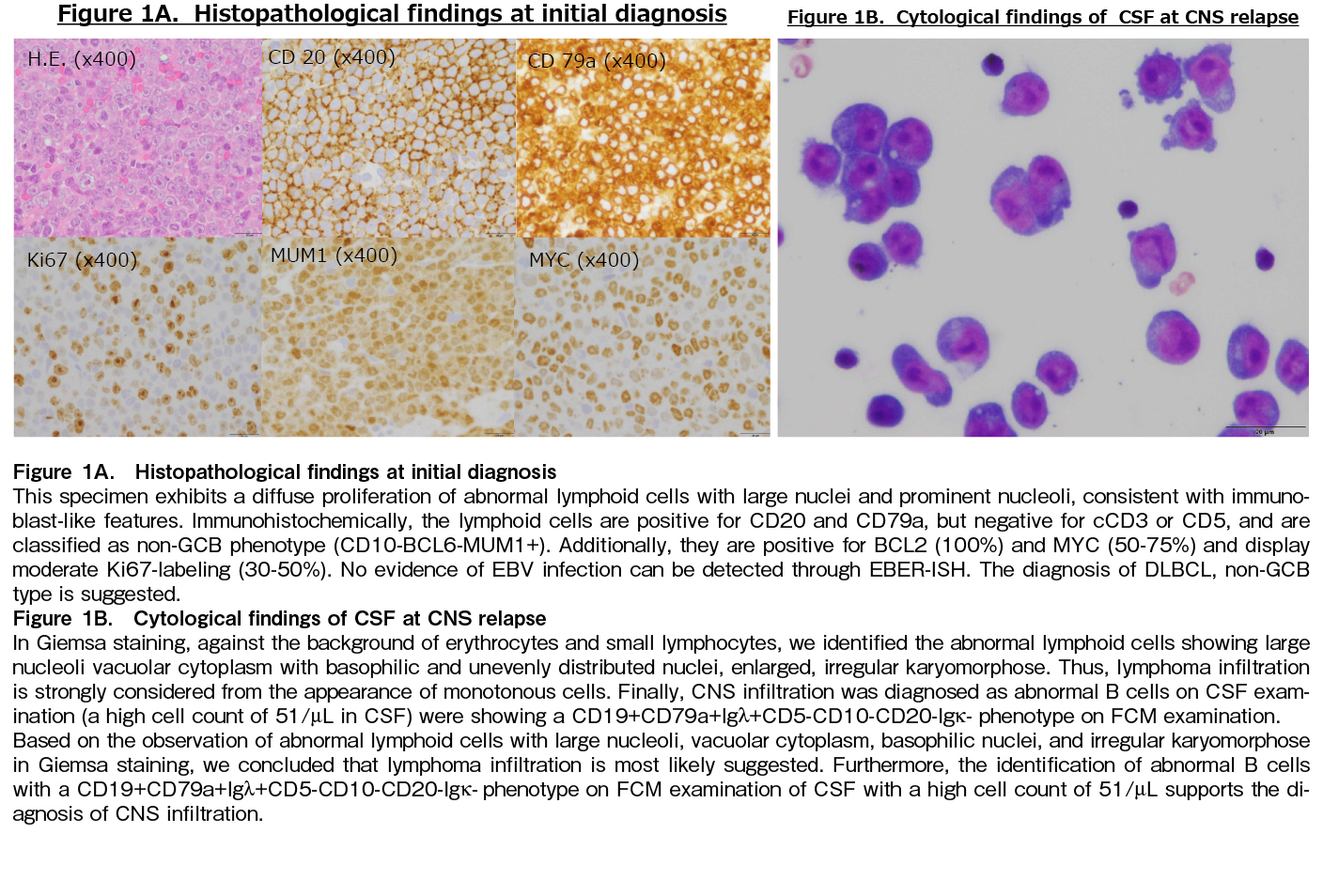
In May 2023, a 66-year-old male was referred to our hospital for further examination of lymph node swelling. Biopsy result revealed non-GC-type DLBCL (Figure 1A) with CS IV, IPI 2, and CNS IPI (intermediate risk). Therefore, we initiated rituximab plus CHOP therapy, leading to complete remission (CR).
However, 3 months after CR, isolated CNS recurrence occurred, with clinical symptoms including ptosis and eye movement disorders. Contrast-enhanced cranial CT or MRI showed no apparent abnormalities. CNS infiltration was confirmed through cerebrospinal fluid (CSF) examination, revealing CD19+CD79a+Igλ+CD5-CD10-CD20-Igκ- phenotype on Flow Cytometry (FCM) examination (Figure 1B). No other systemic lymph nodes lesions were observed. Therefore, high-dose methotrexate (HD-MTX) therapy with intrathecal administration of methotrexate (IT-MTX) was administered, leading to a significant reduction in CSF cell count and a cytology diagnosis of
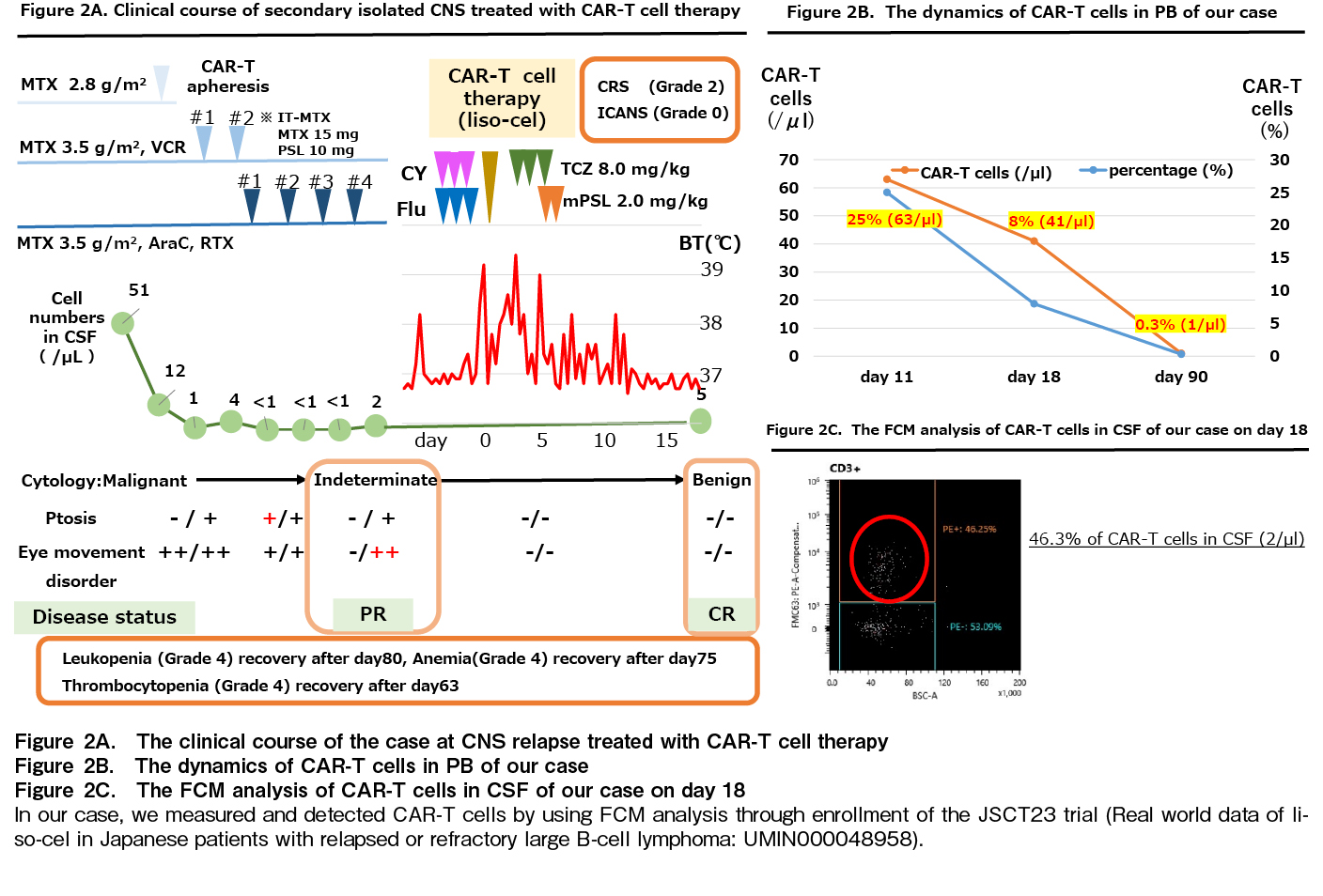
Secondary isolated CNS relapse has a poor prognosis1; therefore, CAR-T cell therapy (liso-cel) was recommended as second-line treatment. The clinical course is presented in Figure 2A. Lymphocytes were harvested using COBE spectra in February 2023, and CAR-T cell therapy was administered in May 2023 with fludarabine (40 mg/body, days 4-2) and cyclophosphamide (300 mg/m2, days 4-2) as pretreatment therapy. CSF examination was performed on day 18 after CAR-T-cell administration indicated CR status with resolution of CNS symptoms. The case was diagnosed with cytokine release syndrome (CRS) (grade 2) according to clinical symptoms, which required tocilizumab administration (8 mg/kg, days 2-4)6. Immune effector cell-associated neurotoxicity syndrome (ICANS) did not occur after CAR-T cell therapy. Although prolonged pancytopenia due to bone marrow failure was observed, hematopoietic function recovery was effectively achieved with G-CSF and eltrombopag combination.
Importantly, the dynamics of CAR-T cells in peripheral blood (PB) were examined using FCM. In PB, 25%, 8%, and 0.3% CAR-T cells were detected on days 11, 18, and 90, respectively (Figure 2B). Residual CAR-T cells were also observed, suggesting that these CAR-T cell populations may contribute to CNS lymphoma control. Notably, we detected 46.3% of CAR-T cells in the CSF (2/μL) on day 18, suggesting that these CAR-T cells can migrate directly to meningeal lesions of DLBCL (Figure 2C).
Discussion
Herein, we validated the efficacy and safety of CAR-T cell therapy (liso-cel) as a second-line therapy for early CNS recurrence of DLBCL in clinical practice.
We compared our case with a case in a previous phase 2 trial5. In the phase 2 trial5, a 54-year-old male was diagnosed with primary testicular lymphoma and intracranial lesions were observed. Therefore, gemcitabine and methotrexate were also administered as salvage therapy. Subsequently, CAR-T cell therapy was initiated and CRS (grade 2) was tolerable with tocilizumab. ICANS and bone marrow failure did not develop after CAR-T cell therapy. Although it led to CR (180 days), the patient later developed PD (day 270). Meanwhile, our case involved a 66-year-old male with early CNS relapse within 1 year. Methotrexate and IT-MTX therapy followed by CAR-T cell therapy resulted in CR on day 120, with grade 2 CRS and no ICANS. Notably, both cases showed a notable effect on CNS lesions, including CNS mass in the previous case and meningeal lesions in the present case.
It is important to discuss whether CAR-T cell therapy is effective in patients with CNS lesions.
In our case of meningeal lesions, aggressive irradiation may be predicted to result in a poor outcome with potential side effects of severe myelosuppression and leukoencephalitis. In our case of early relapse after R-CHOP therapy, autologous peripheral blood stem cell transplantation might have provided only a temporary effect with poor outcomes due to the short period from the last chemotherapy to DLBCL relapse (<1 year). Therefore, CAR-T cell therapy may be a suitable second-line treatment option for our case.
However, the risk of migration to the CNS and ICANS occurrence remains. Recent reports have shown that CAR-T cells are continuously detected in the CSF after CAR-T cell therapy7; these cells typically migrate to tumor sites8. Therefore, among CAR-T cell therapies2–5, liso-cel has been approved by Japanese insurance for CNS lymphoma based on the results of the global phase 2 trial (TRANCEND NHL 001), showing 50% of overall response rate (ORR) (3/6) in six Japanese patients5. In our case, CAR-T cell therapy was an appropriate indication because there was no problem with the use of liso-cel as a second-line treatment for early recurrence after R-CHOP and active SCNSL.
First, in terms of efficacy, the sequential analysis of CAR-T cells in PB showed a high peak of CAR-T cells on day 11 and residual CAR-T cells on day 90, suggesting immune surveillance of CNS lymphoma. Herein, we compared a previous report on CAR-T cell dynamics (Hu K's report, 2021) with our case. According to the analysis of PB and CSF of CAR-T cells in B-ALL, CAR-T cells in CSF also increased proportionally to the increase in CAR-T cells in PB9, the peak of PB was 6% and the transition to CSF was 20%, respectively9. Therefore, the PB peak in our case was 25%, and the CSF transition may be speculated. We detected 46.3% CAR-T cells in the CSF on day 18, suggesting that these CAR-T cells can directly infiltrate CNS lesions. Furthermore, long-term CAR-T cell retention may contribute to disease control.
Second, in our case, CRS was tolerated with tocilizumab treatment. ICANS did not occur after CAR-T cell therapy. In a phase 2 study, CRS (grade 2) was also treated with tocilizumab and ICANS was absent5. Based on only two cases in Japan, the safety of CRS and ICANS was not severe, contrary to previous expectations5.
Finally, we present a remarkable meta-analysis of CAR-T therapy for CNS lymphoma (15 studies including 30 PCNSL and 98 SCNSL cases), showing an excellent ORR (64%; primary CNS lymphoma, 57%; secondary CNS lymphoma) and safety of ICANS (48%) (26%; grade 3-4)10.
In conclusion, we validated the efficacy and safety of CAR-T-cell therapy for early CNS recurrence in patients with DLBCL. More cases treated with CAR-T cell therapy for CNS lymphoma will be needed.
Acknowledgments
We thank the medical and nursing staff who cared for the patient. Furthermore, we thank Naoko Ban for the technical support of FCM analysis in CAR-T cells.
Informed consent
The informed consent was obtained from the patient in our case report.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
Acknowledgments
We thank the medical and nursing staff who cared for the patient. Furthermore, we thank Naoko Ban for the technical support of FCM analysis in CAR-T cells.
Author Contributions
KT, NK, YM and TY have contributed equally to this manuscript. TT, TN, KY, KM, IK, KM, KO, KK, KA cared for the patient. KM, KO, and KA supervised the case presentation and discussion. KM, KM, MS performed FCM analysis of CAR-T cells in PB and CSF.
Acknowledgments
We thank the medical and nursing staff who cared for the patient. Furthermore, we thank Naoko Ban for the technical support of FCM analysis in CAR-T cells.
The approval by the Institutional Review Board (IRB)
This case report was approved as 23-15 by the IRB.
References
1.Kawano N, Ochiai H, Yoshida S, Yamashita K, Shide K, Shimoda H, et al. Clinical features and treatment outcomes of isolated secondary central nervous system lymphomas in Miyazaki Prefecture. Int J Clin Oncol. 2012; 17: 336-40.
2.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucal CAR T-cell therapy in refactory diffuse large B-cell lymphoma. N Engl J Med. 2017; 377: 2531-44.
3.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refactory diffuse large B-cell lymphoma. N Engl J Med. 2019; 380: 45-56.
4.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refactory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020; 396: 839-52.
5.Makita S, Yamamoto G, Maruyama D, Asano-Mori Y, Kaji D, Ananthakrishnan R, et al. Phase 2 results of lisocabtagene maraleucel in Japanese patients with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Cancer Med. 2022; 11: 4889-99.
6.Proter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol. 2018; 11: 35.
7.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeri antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013; 368: 1509-18.
8.Keu KV, Witney TH, Yaghoubi S, Rosenberg J, Kurien A, Magnusson R, et al. Reportor gene imaging of targeted Tcell immunotherapy in recirrent glioma. Sci Trransl Med. 2017; 9: eaag2196.
9.Hu K, Wang Y, Teng X, Hu Y, Huang H. Cell subsets and cytokine dynamics in cerebrospinal fluid after CAR-T cell therapy for B-cell acute lymphoblastic leukemia with central nervous system involvement. Bone Marrow Transplant. 2021; 56: 3088-90.
10.Cook MR, Dorris CS, Makambi KH, Luo Y, Munshi PN, Donato M, et al. Toxicity and efficacy of CAR T-cell therapy in primary and secondary CNS lymphoma: a meta-analysis of 128 patients. Blood Adv. 2023; 7: 32-39.
Search
News