Volume 6 (2023) Issue 4 No.8 Pages 151-157
Abstract
Hematopoietic stem cells (HSCs) are a rare cell population present in the bone marrow. They possess self-renewal and multipotent differentiation capacities and play a crucial role in lifelong hematopoiesis and reconstitution of the hematopoietic system after hematopoietic stem cell transplantation (HSCT). HSCT remains the only curative treatment for refractory hematologic disorders. Umbilical cord blood (CB) has several advantages as an alternative donor for HSCT, including HLA flexibility and lack of donor burden. However, CB has limitations in terms of cell dose, restricted donor options, and prolonged time to engraftment. Development of techniques for expanding HSCs ex vivo, especially those contained in CB, has become a goal in the field of hematology. Attempts have been made to use various combinations of cytokines for this purpose, but these protocols showed limited expansion rates and did not progress to clinical applications. Recent advances that include the addition of small molecules to cytokines have enabled long-term and stable ex vivo expansion of human HSCs. Clinical trials have been conducted with HSCs expanded in CB using these techniques, confirming their efficacy and safety. Furthermore, we recently developed a recombinant cytokine-free, albumin-free culture system for long-term expansion of human HSCs. This approach has the potential to selectively expand human HSCs more effectively than the previous protocols. We herein present an overview of ex vivo culture protocols for expanding human HSCs together with the results of clinical trials that utilized these techniques.
Introduction
Hematopoietic stem cells (HSCs) constitute a rare cell population residing in the bone marrow; they possess self-renewal and multipotent differentiation capacities that sustain lifelong hematopoiesis1. HSCs play a pivotal role in the curative treatment of various refractory hematologic disorders through hematopoietic stem cell transplantation (HSCT). HSCs are also present in the umbilical cord blood (CB), which is now commonly used as a source of stem cells2. CB offers advantages such as greater tolerance for human leukocyte antigen (HLA) disparities and lack of burden on donors. However, the limited number of HSCs in CB not only restricts the CB units that can be transplanted but also results in delays in engraftment and increases the risk of infection3. Therefore, ex vivo expansion of umbilical CB-derived HSCs holds significant importance for enhancing the safety of transplantation procedures. This review focuses on recent advancements in ex vivo expansion techniques for human HSCs and their application in clinical research, which have sought to address these challenges and promote safer transplantation practices.
Cytokines and Growth Factors
By combining insights into HSC self-renewal in vivo, researchers have developed various techniques for ex vivo HSC expansion. Central to this process are cytokines and growth factors in widely used combinations, including stem cell factor (SCF), thrombopoietin (TPO), Fms-like tyrosine kinase 3 ligand (FLT3-L), and interleukin-6 (IL-6). SCF, initially identified as a c-Kit (CD117) ligand in mice4, has been shown to play a pivotal role in HSC survival and expansion5. Within the bone marrow niche, SCF expression promotes HSC maintenance and stimulates cell cycle progression via activation of the phosphoinositide 3-kinase (PI3K)/AKT/FOXO pathway6. TPO is essential for HSC maintenance and expansion and induces self-renewing cell division. By signaling through the MPL receptor, TPO triggers multiple pathways, including JAK/STAT, MAPK/ERK, and PI3K/AKT7, 8. FLT3-L and IL-6, too, contribute to cell amplification via diverse signaling pathways9, 10. Despite these advances, cytokine-based culture systems have not achieved long-term stable HSC self-renewal and their full-scale clinical application remains unrealized11, 12.
Small Molecules
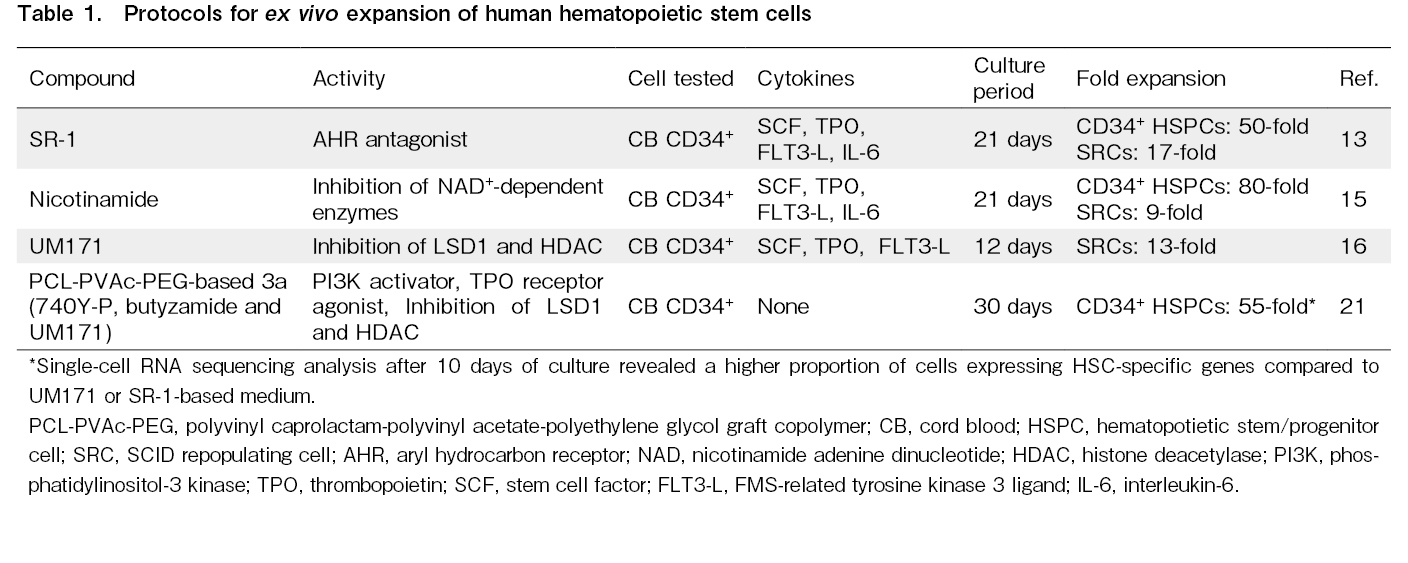
While the expansion of human HSCs using cytokine combinations alone has limitations, recent studies have indicated that the addition of small molecules to these cytokines can enhance expansion efficiency (Table 1).
The front-line compound in this field, StemRegenin-1 (SR-1), was identified by screening a library of 100,000 compounds designed to detect molecules that enhance the proliferation of umbilical CB-derived hematopoietic stem and progenitor cells (HSPCs)13. SR-1 is a purine derivative and an antagonist of the aryl hydrocarbon receptor (AHR) of human HSPCs. When SR-1 was added to cytokines in a culture of umbilical CB CD34+ cells, the cells expanded 50-fold and SCID-repopulating cells (SRCs) that sustain long-term hematopoiesis in immunodeficient mice were amplified 17-fold. The precise molecular mechanism underlying the action of SR-1 is not fully understood. However, overexpression of the RNA-binding protein Musashi-2 (MSI2) has been suggested to directly suppress the AHR signal and thus expand HSCs ex vivo14.
In 2012, a breakthrough was achieved by combining nicotinamide (NAM), a form of vitamin B-3, with cytokines to enhance the expansion of umbilical CB-derived CD34+ cells. This approach led to 80-fold expansion of these cells within three weeks15. Xenotransplantation assays showed that the addition of NAM led to a 9-fold increase in SRCs, enhancing bone marrow homing via the CXCR4-CXCL12 pathway. These grafts allowed for hematopoietic reconstitution using human cells of both myeloid and lymphoid lineages. NAM functions as an inhibitor of NAD+-dependent enzymes, particularly sirtuin, which play a central role in the proliferation of human HSCs.
In 2014, through the screening of compound libraries, a pyrimidoindole derivative, UM171, was identified to expand human HSCs when used in combination with cytokines in culture16. In xenotransplantation assays, a 13-fold increase in SRCs was observed. Recent research has revealed that UM171 activates the CULLIN3-E3 ubiquitin ligase (CRL3) complex, which includes KBTBD4, to ubiquitinate the CoREST complex. As a result, lysine-specific histone demethylase 1 (LSD1) and histone deacetylases (HDACs) within the CoREST complex are inactivated, suggesting that ubiquitination suppresses genes involved in differentiation17. Functional and genomic studies have suggested that UM171 acts independent of AHR inhibition, suggesting a synergistic increase in the amplification efficiency of HSCs when used in combination with SR-1.
Polymers and Chemicals
HSC culture has traditionally required the presence of bovine serum albumin (BSA) or fetal bovine serum (FBS) together with cytokines. Albumin is considered to play a crucial role in HSC culture as a carrier protein or source of amino acids. However, it is difficult to obtain stable results due to significant variations between batches. This instability and uncertainty pose significant obstacles to clinical applications. In 2017, synthetically produced albumin extracted through yeast culture was reported to exhibit functionality equivalent to that of albumin extracted from serum, with reduced batch-to-batch variation and stable outcomes18. However, even with synthetic albumin, trace amounts of yeast impurities were unavoidable.
Wilkinson et al. conducted screening for chemically synthesized substances that could be substituted for albumin. By adding SCF and TPO to a medium containing polyvinyl alcohol (PVA), they determined that it was possible to amplify mouse HSCs ex vivo without the addition of albumin19. Surprisingly, after one month of culture under these conditions, the number of functional HSCs increased 236- to 899-fold. PVA not only acted as a suitable substitute for albumin but also achieved even greater amplification of HSCs. There are various types of PVA with different molecular weights and degrees of hydrolysis. Sudo et al. reported that PVA with a lower degree of hydrolysis efficiently amplifies both mouse and human HSCs, while the molecular weight has no impact on the ex vivo expansion of HSCs20. PVA can be synthesized stably and inexpensively in large quantities, offering advantages in terms of cost compared with the use of animal-derived components or synthetic proteins in cultures. However, the efficiency of human HSC expansion remains limited under the same culture conditions.
To investigate the differences between murine and human HSPCs, we analyzed the phosphorylation status of major signaling pathways in cultured HSPCs21. The results demonstrated a significant decrease in the PI3K/AKT pathway in humans. To activate the PI3K/AKT pathway, we added the PI3K activator 740Y-P and obtained improved expansion rates of human HSPCs on day 7. In addition, we found that SCF could be substituted with 740Y-P, and TPO with the TPO receptor agonist butyzamide22, 23. We confirmed that a medium containing PVA, 740Y-P, and butyzamide (2a medium) allowed for a 7-day culture of human HSPCs without the need for cytokines. However, using this approach, HSPCs began to differentiate into megakaryocytes after 14 days. To achieve long-term stable culture, we explored compounds that could prevent the differentiation of HSCs. We discovered that by adding the pyrimidoindole derivative UM171 to the 2a medium (3a medium), human HSCs could be cultured stably for up to 30 days. We also confirmed that the capacity for hematopoietic reconstruction was maintained by transplanting cells cultured in 3a medium into immunodeficient mice after irradiation.
To further improve expansion efficiency, we conducted screening for polymers and determined that the polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer (PCL-PVAc-PEG)24, 25 possesses superior cell proliferation potential compared to PVA. When 740Y-P, butyzamide, and UM171 were added to culture medium containing PCL-PVAc-PEG instead of PVA, the proliferation capacity of human HSPCs was further enhanced. Over 30 days, the total cell count increased 75-fold and CD34+ cells expanded 55-fold. Transplanting these cells after a 30-day culture into immunodeficient mice confirmed their engraftment. Moreover, robust human CD45 chimerism was observed in secondary xenotransplantation recipients.
To investigate the characteristics of cells cultured in the PCL-PVAc-PEG-based 3a medium, we conducted single-cell RNA sequencing analysis. This analysis revealed that a high proportion of the cells expressed genes specific to HSCs. Notably, this proportion was higher than those reported in recent clinical trials involving UM171 or SR-1 for human HSC culture. These findings suggest that the PCL-PVAc-PEG-based 3a medium achieves selective expansion of HSCs. This medium holds some advantages, as it does not contain recombinant proteins and is chemically defined, resulting in improved batch-to-batch consistency, reduction in reagent costs, and acceleration of clinical applications.
Clinical Applications
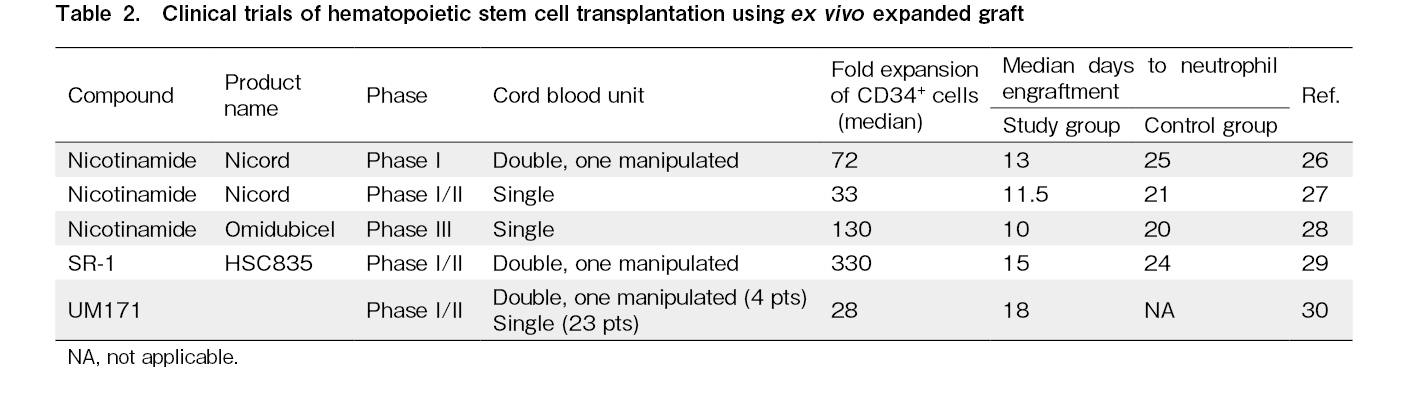
Clinical trials using ex vivo-expanded CB have already shown promising results (Table 2). Among these approaches, NAM is pioneering. In a Phase I trial, double CB transplantation was performed on 11 patients with hematologic malignancies after myeloablative conditioning26. This procedure involved simultaneous transplantation of NAM-expanded CB, NiCord (Gamida Cell, Israel), and unmanipulated umbilical CB. NiCord is a product obtained by culturing CD133+ cells from umbilical CB in a medium containing NAM for 21 days and then adding unmanipulated T cells purified from the CD133− fraction. After three-week culture, CD34+ cells expanded by a median of 72-fold. No severe adverse events related to NiCord infusion were observed. The median time to neutrophil engraftment in the NiCord transplantation group was significantly shorter than that in the historical control group (13 vs 25 days). Complete or partial engraftment of neutrophils and T cells derived from NiCord was observed in eight patients, and the engraftment of NiCord remained stable in all patients throughout the median follow-up period of 21 months.
Next, a phase I/II clinical trial involving the transplantation of NiCord alone was conducted after myeloablative conditioning in 36 patients with hematologic malignancies27. The culture with NAM resulted in a 33-fold expansion of CD34+ cells, and the median time to neutrophil engraftment after NiCord transplantation was significantly shorter than that in the historical control group (11.5 vs 21 days). For the patients who achieved platelet recovery, the median time to platelet recovery was 34 days for the NiCord group and 46 days for the historical control group, indicating significant improvement in the NiCord group. One patient experienced primary graft failure and two patients experienced secondary graft failure. There was no significant difference in the occurrence of grade III-IV acute graft-versus-host disease (GVHD) or chronic GVHD between the two groups. Moreover, no significant difference was observed in the 2-year disease-free survival (DFS) or overall survival (OS) after transplantation.
Subsequently, a phase III randomized controlled trial was conducted28. Eligible patients were aged 12 to 65 years with high-risk hematologic malignancies, were candidates for myeloablative allogeneic HSCT, and lacked immediately available matched sibling or unrelated donors. A total of 125 patients were enrolled, of whom 62 were assigned to the omidubicel (formerly NiCord) group and 63 to the conventional umbilical CB transplantation group. There were no significant differences in the patient characteristics. CD133+ fractions were cultured for 21±2 days with NAM, and transplanted along with CD133− cell fractions, including T cells. After culture, CD34+ cells were expanded approximately 130-fold (range, 32-233 fold), resulting in a CD34+ cell count of 9.0 (range, 2.1-47.6) × 106 cells/kg in the omidubicel group and 0.3 (range, 0.1-1) × 106 cells/kg in the control group. The median time to neutrophil engraftment was significantly shorter in the omidubicel group (12 vs 22 days). Among the patients receiving omidubicel, higher total CD34+ cell counts and CD34+ cell doses (per weight) correlated with a shorter time to neutrophil engraftment. In addition, platelet engraftment rates up to day 42 were significantly higher in the omidubicel group (55% vs 35%). The cumulative incidence of bacterial or invasive fungal infections was significantly lower in the omidubicel group (37% vs 57%) and the number of days spent outside the hospital within 100 days post-transplantation was significantly higher in the omidubicel group (61 vs 48 days). There were no significant differences in the cumulative incidence of grade III-IV acute GVHD or chronic GVHD between the groups. However, despite the lack of statistical significance, favorable trends were observed in terms of the cumulative incidence of non-relapse mortality (NRM), DFS, and OS in the omidubicel group. Based on these results, on April 17, 2023, the U.S. Food and Drug Administration (FDA) approved omidubicel-onlv (marketed as Omisirge by Gamida Cell Ltd.) for adult and pediatric patients (12 years and older) with hematologic malignancies scheduled for umbilical CB transplantation after myeloablative conditioning; the aim is to reduce neutrophil recovery time and infection rates. This represents a significant step in the ex vivo expansion technology of human umbilical CB.
In 2016, the results of a phase I/II trial involving expanded CB transplantation using SR-1 were reported29. In this clinical trial, a product called HSC835 was used, which involved culturing CD34+ cells from CB in a culture medium containing SR-1 for 15 days and then adding cryopreserved CD34− fraction cells. Double CB transplantation was performed by transplanting HSC835 and another unmanipulated CB unit. Seventeen patients who underwent myeloablative conditioning received this product. The CD34+ cells expanded approximately 330-fold throughout the culture period. All patients achieved engraftment. The median time to neutrophil engraftment was significantly shorter in the HSC835 group than in the historical control group (15 vs 24 days), and the median time to platelet engraftment, too, was significantly shorter (49 vs 89 days). Peripheral blood chimerism analysis revealed that the engrafted cells of the six individuals were entirely derived from HSC835, while those of another six individuals were entirely derived from the unmanipulated umbilical CB unit. Those of the remaining five patients exhibited a unique chimerism pattern in which myeloid cells originated from HSC835, while T cells originated from the unmanipulated umbilical CB unit. Although no significant differences were observed in terms of the incidence of acute GVHD, NRM, or OS compared to the historical control group, hospitalization duration was significantly shorter in the HSC835 group (30 vs 46 days).
In 2020, the results of a phase I/II trial utilizing UM171 were reported30. This clinical trial targeted patients with hematologic malignancies who lacked a suitably matched HLA donor. The trial involved transplantation of umbilical CB-derived CD34+ cells cultured for 7 days in a medium containing UM171, along with a
The trial aimed to determine the minimum umbilical CB cell dose required for achieving engraftment. The expansion process revealed that a pre-culture CD34+ cell count of 0.52×105/kg was sufficient. Based on these results, patients weighing 70 kg who were previously eligible for only 5% of the available umbilical CB units based on conventional criteria could now be eligible for 47% of the units based on the criteria established in this clinical trial. The authors concluded that the UM171 single umbilical CB protocol offers the clinical benefits of faster engraftment and reduced early infection complications, while also allowing for the use of HLA-matched umbilical CB units with lower total cell counts through an expanded approach.
Recent advancements in the culture protocols of human HSCs have not only enabled the substantial expansion of human HSCs but have also led to promising results in clinical trials. Clinical evaluations of ex vivo umbilical CB expansion have confirmed the safety and rapid recovery of neutrophils and platelets, which are associated with favorable early clinical outcomes post-transplantation. However, long-term clinical benefits have not yet been directly demonstrated in comparative clinical trials.
Regarding immune reconstitution, the median number of infused T cells was 2-3 times lower in UM171-expanded CB than in unmanipulated CB, even though the quantity and phenotype of T cells after transplantation were similar between the two groups31. T cell receptor sequencing analysis indicated that the patients who received UM171-expanded CB displayed increased T cell diversity and rapid virus-specific T cell responses at 12 months post-transplantation, leading to a significant reduction in the occurrence of severe infection. Furthermore, in the case of omidubicel, immune reconstitution was comparable to that achieved using unmanipulated CB32.
To evaluate the impact of expanded CB on prognosis, a retrospective comparison was conducted between the results of clinical trials utilizing UM171-expanded CB and outcomes using non-manipulated CB and matched unrelated donor (MUD) transplantation33. The results revealed that the UM171 group exhibited lower NRM compared to the CB control group, along with improved 2-year graft-versus-host disease-free relapse-free survival (GRFS) and 1-year OS. Furthermore, compared to the MUD-peripheral blood stem cell (PBSC) group, the UM171 group experienced fewer cases of grade III-IV acute GVHD and chronic GVHD. These results suggest that recipients of UM171-expanded CB may benefit from reduced NRM and improved GRFS. In addition, a meta-analysis of clinical trials utilizing expanded CB indicated a lower risk of death at the study endpoint for patients who underwent ex vivo expansion34.
Conclusion
Collectively, progress in ex vivo expansion techniques for HSCs, coupled with accumulating clinical evidence, makes expanded CB transplantation a feasible option in clinical practice. Further mature results are eagerly anticipated.
Author Contributions
M.S. conceptualized, wrote, and edited the manuscript.
Conflicts of Interest
M.S. is co-founder and shareholder in Celaid Therapeutics. Disclosure form provided by the author is available on the website.
References
1.Wilkinson AC, Igarashi KJ, Nakauchi H. Haematopoietic stem cell self-renewal in vivo and ex vivo. Nat Rev Genet. 2020; 21: 541-54.
2.Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989; 321: 1174-8.
3.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013; 122: 491-8.
4.Williams DE, Eisenman J, Baird A, Rauch C, Van Ness K, March CJ, et al. Identification of a ligand for the c-kit proto-oncogene. Cell. 1990; 63: 167-74.
5.Bernstein ID, Andrews RG, Zsebo KM. Recombinant human stem cell factor enhances the formation of colonies by CD34+ and CD34+lin- cells, and the generation of colony-forming cell progeny from CD34+lin- cells cultured with interleukin-3, granulocyte colony-stimulating factor, or granulocyte-macrophage colony-stimulating factor. Blood. 1991; 77: 2316-21.
6.Yamazaki S, Iwama A, Takayanagi S, Morita Y, Eto K, Ema H, et al. Cytokine signals modulated via lipid rafts mimic niche signals and induce hibernation in hematopoietic stem cells. EMBO J. 2006; 25: 3515-23.
7.Yamada M, Komatsu N, Okada K, Kato T, Miyazaki H, Miura Y. Thrombopoietin induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in a human thrombopoietin-dependent cell line. Biochem Biophys Res Commun. 1995; 217: 230-7.
8.Miyakawa Y, Oda A, Druker BJ, Kato T, Miyazaki H, Handa M, Ikeda Y. Recombinant thrombopoietin induces rapid protein tyrosine phosphorylation of Janus kinase 2 and Shc in human blood platelets. Blood. 1995; 86: 23-7.
9.Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018; 15: 234-48.
10.Masson K, Rönnstrand L. Oncogenic signaling from the hematopoietic growth factor receptors c-Kit and Flt3. Cell Signal. 2009; 21: 1717-26.
11.Bhatia M, Bonnet D, Kapp U, Wang JC, Murdoch B, Dick JE. Quantitative analysis reveals expansion of human hematopoietic repopulating cells after short-term ex vivo culture. J Exp Med. 1997; 186: 619-24.
12.Conneally E, Cashman J, Petzer A, Eaves C. Expansion in vitro of transplantable human cord blood stem cells demonstrated using a quantitative assay of their lympho-myeloid repopulating activity in nonobese diabetic-scid/scid mice. Proc Natl Acad Sci U S A. 1997; 94: 9836-41.
13.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010; 329: 1345-8.
14.Rentas S, Holzapfel N, Belew MS, Pratt G, Voisin V, Wilhelm BT, et al. Musashi-2 attenuates AHR signalling to expand human haematopoietic stem cells. Nature. 2016; 532: 508-11.
15.Peled T, Shoham H, Aschengrau D, Yackoubov D, Frei G, Rosenheimer NG, et al. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol. 2012; 40: 342-55.e1.
16.Fares I, Chagraoui J, Gareau Y, Gingras S, Ruel R, Mayotte N, et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014; 345: 1509-12.
17.Subramaniam A, Žemaitis K, Talkhoncheh MS, Yudovich D, Bäckström A, Debnath S, et al. Lysine-specific demethylase 1A restricts ex vivo propagation of human HSCs and is a target of UM171. Blood. 2020; 136: 2151-61.
18.Ieyasu A, Ishida R, Kimura T, Morita M, Wilkinson AC, Sudo K, et al. An all-recombinant protein-based culture system specifically identifies hematopoietic stem cell maintenance factors. Stem Cell Reports. 2017; 8: 500-8.
19.Wilkinson AC, Ishida R, Kikuchi M, Sudo K, Morita M, Crisostomo RV, et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature. 2019; 571: 117-21.
20.Sudo K, Yamazaki S, Wilkinson AC, Nakauchi H, Nakamura Y. Polyvinyl alcohol hydrolysis rate and molecular weight influence human and murine HSC activity ex vivo. Stem Cell Res. 2021; 56: 102531.
21.Sakurai M, Ishitsuka K, Ito R, Wilkinson AC, Kimura T, Mizutani E, et al. Chemically defined cytokine-free expansion of human haematopoietic stem cells. Nature. 2023; 615: 127-33.
22.Sakurai M, Takemoto H, Mori T, Okamoto S, Yamazaki S. In vivo expansion of functional human hematopoietic stem progenitor cells by butyzamide. Int J Hematol. 2020; 111: 739-41.
23.Nogami W, Yoshida H, Koizumi K, Yamada H, Abe K, Arimura A, et al. The effect of a novel, small non-peptidyl molecule butyzamide on human thrombopoietin receptor and megakaryopoiesis. Haematologica. 2008; 93: 1495-504.
24.Jin X, Zhou B, Xue L, San W. Soluplus? micelles as a potential drug delivery system for reversal of resistant tumor. Biomed Pharmacother. 2015; 69: 388-95.
25.Linn M, Collnot EM, Djuric D, Hempel K, Fabian E, Kolter K, et al. Soluplus? as an effective absorption enhancer of poorly soluble drugs in vitro and in vivo. Eur J Pharm Sci. 2012; 45: 336-43.
26.Horwitz ME, Chao NJ, Rizzieri DA, Long GD, Sullivan KM, Gasparetto C, et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest. 2014; 124: 3121-8.
27.Horwitz ME, Wease S, Blackwell B, Valcarcel D, Frassoni F, Boelens JJ, et al. Phase I/II study of stem-cell transplantation using a single cord blood unit expanded ex vivo with nicotinamide. J Clin Oncol. 2019; 37: 367-74.
28.Horwitz ME, Stiff PJ, Cutler C, Brunstein C, Hanna R, Maziarz RT, et al. Omidubicel vs standard myeloablative umbilical cord blood transplantation: results of a phase 3 randomized study. Blood. 2021; 138: 1429-40.
29.Wagner JE, Brunstein CG, Boitano AE, DeFor TE, McKenna D, Sumstad D, et al. Phase I/II trial of StemRegenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cel. 2016; 18: 144-55.
30.Cohen S, Roy J, Lachance S, Delisle JS, Marinier A, Busque L, et al. Hematopoietic stem cell transplantation using single UM171-expanded cord blood: a single-arm, phase 1-2 safety and feasibility study. Lancet Haematol. 2020; 7: e134-45.
31.Dumont-Lagacé M, Li Q, Tanguay M, Chagraoui J, Kientega T, Cardin GB, et al. UM171-Expanded cord blood transplants support robust T cell reconstitution with low rates of severe infections. Transplant Cell Ther. 2021; 27: 76.e1-9.
32.Szabolcs P, Mazor RD, Yackoubov D, Levy S, Stiff P, Rezvani A, et al. Immune reconstitution profiling suggests antiviral protection after transplantation with Omidubicel: a phase 3 substudy. Transplant Cell Ther. 2023; 29: 517.e1-12.
33.Cohen S, Bambace N, Ahmad I, Roy J, Tang X, Zhang MJ, et al. Improved outcomes of UM171-expanded cord blood transplantation compared with other graft sources: real-world evidence. Blood Adv. 2023; 7: 5717-26.
34.Saiyin T, Kirkham AM, Bailey AJM, Shorr R, Pineault N, Maganti HB, et al. Clinical outcomes of umbilical cord blood transplantation using ex vivo expansion: a systematic review and meta-analysis of controlled studies. Transplant Cell Ther. 2023; 29: 129.e1-9.
Search
News